Numerical Sequence of Borane Series
Enos Masheija Kiremire
Department of Chemistry and Biochemistry, University of Namibia, Private Bag 13301, Windhoek, Namibia
DOI : http://dx.doi.org/10.13005/ojc/300317
Article Received on :
Article Accepted on :
Article Published : 18 Sep 2014
A table of hydroborane families has been created. The table links boranes of different families(homologous series) and members of the same family based on k number. The table is useful deducing straight away whether a borane( molecular formula) is closo, nido or arachno or something else. The table also indicates that boranes are formed according to natural periodic function (arithmetical progression). The empirical formula utilized is extremely versatile, simple and based on the principle of Nobel gas configuration. It could be used in both simple and complex boranes and carboranes. The closo members which portray characteristic shapes also have characteristic k1 numbers.
KEYWORDS:Numerical Sequence; hydroborane; number
Download this article as:| Copy the following to cite this article: Kiremire E. M. Numerical Sequence of Borane Series. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Kiremire E. M. Numerical Sequence of Borane Series. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4768 |
INTRODUCTION
As a result of the carbon (C) atom to catenate and also form strong bonds with a hydrogen atom, it is able to generate a vast range of families of hydrocarbons. Some of the common families may be given by the formulas
F1 = CnH2n+2 (alkanes), F2 = CnH2n (alkenes), and F3 = CnH2n-2 (alkynes).
For each of these families, the carbon atom obeys the octet (8) rule which has its base in a noble gas configuration of neon (Ne). On the other hand the boron atom (B) which is next to the carbon atom one valence electron less, mimics the carbon atom to produce boron hydrides (boranes) in an attempt to obey the octet rule. For instance, diborane B2H6 = (BH)2H4 may regarded as attempting to mimic C2H4 since (BH) fragment is electronically equivalent to (C) atom. Indeed, their shapes could be viewed as indicated in the sketch of Fig. 1. The two hydrogen atoms indicated by dots in the two bonds, can be considered to donate two electrons into the diboranemolecule so as to enable the boron atoms to satisfy the octet rule. In reality, it should be noted that diborane has two banana bonds of bridging H atoms.
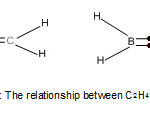 |
Figure 1 A Click here to View Figure |
BORANE SERIES
Just like hydrocarbons, the known boron hydrides have been grouped into families. These families are known as CLOSO, NIDO, ARACHNO and HYPO and the rare one KLADO. These families may be represented by the formulas given below.
G0 = BnHn , G1 = BnHn+2 (CLOSO), G2 = BnHn+4(NIDO) , G3 = BnHn+6 (ARACHNO) , G4 = BnHn+8 (HYPO), G5 = BnHn+10 (P =KLADO), G6 = BnHn+12 (Q),G7 = BnHn+14(R). These series (families) are given in Table 1. Each member of the family has been given a number (k1 value). The k1 values are calculated from the empirical formula k1 = ½ (E-V), where E = sum of octet electrons and V = sum of valence electrons in the cluster. This empirical formula has been discussed in our previous publications1-2. What is interesting about the empirical formula is that it predicts a quadruple bond for C2 diatomic molecule as found from high level theoretical calculation methods3-4.
ANALYSIS OF k1 VALUES
The k1 numbers do vary from family to family and also within the members of the same boron family. A movement from one family member to the next family member (horizontally, similar to a period in the periodic table), the k1 number varies by 1. On the other hand a movement from one member to the next member of the same family( similar to a group in the periodic table), the k1 number varies by 2. Clearly these are simple arithmetical series. The following examples illustrate this point.
been given a number (k1 value). The k1 values are calculated from the empirical formula k1 = ½ (E-V), where E = sum of octet electrons and V = sum of valence electrons in the cluster. This empirical formula has been discussed in our previous publications1-2. What is interesting about the empirical formula is that it predicts a quadruple bond for C2 diatomic molecule as found from high level theoretical calculation methods3-4.
Across families: B6H6(12), B6H8(11), B6H10(10), B6H12(9), B6H14(8); (simply add or subtract 2H atoms)and across members of the same family: B5H7(9), B6H8(11), B7H9(13), B8H10(15), B9H11(17), B10H12(19), B11H13(21), B12H14(23)( simply add or subtract BH fragment). A large number of hypothetical neutral boranes expected from the formulas have never been synthesized. For instance, the neutral members of the closo family, B5H7, B6H8, B7H9, B8H10, B9H11, B10H12, B11H13, and B12H14 have not been observed instead B5H5⏋2―, B6H6⏋2―, B7H7⏋2―, B8H8⏋2―, B9H9⏋2―, B10H10⏋2―, B11H11⏋2―, and B12H12⏋2―, are well known. In Table 1, it hasbeen found easier to explain the series by assuming that all boranes are neutral. For instance to test to which family a hydroborane belongs, B2H7⏋― is regarded as B2H8 and B5H8⏋― as B5H9. A collection sample of boranes have been classified based on Table 1 and these are presented in Table 5.
DIAGONAL RELATIONSHIP WITHIN THE FAMILIES
The diagonal relationship within boranec families can readily be discerned from Table 1. The examples include B5H7(9)→ B4H8(6)→ B3H9(3); and B6H8(11)→ B5H9(8)→ B4H10(5). This diagonal relationship also presented horizontally in Table 3, is cited widely in form of shapes5 and is partially presented in Fig. 1. The k1 numbers vary by 3 units. The diagonal relationship within boron families when set up horizontally, another diagonal relationship is detected. The variations are given in Table 4. This relationship links up boranes with the same number of B atoms but different number of hydrogen atoms from different families. For instance, B5H7(9)(CLOSO)→ B5H9(8)(NIDO)→ B5H11(7)(ARACHNO). The k1values vary by 1 and therefore the shapes differ.It has also been found from theoretical studies by Yao and Hoffman6 that the boranes B3H9 , B4H12 and B6H18 have some stability with B6H18 being the most stable. These boranes belong to the families, ARACHNO, HYPO, and P (see Table 1) respectively. Furthermore, theoretical calculations7 indicate that the boranesB4H2, B4H24, B4H6, B4H8 and B4H10( belong to the families B,C, N, AR in the Table 1) are more stable than B4H, B4H3, B4H5, B4H7, and B4H9 which do not belong to any of the families.
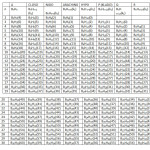 |
Table1: The k1 Values and the Borane Series Click here to View table |
 |
Table2: Diagonal Relationship of k Values ofBoranes for n =2 to 12 Click here to View table |
 |
Table3: Geometrical Relationship corresponding to the Diagonal Relationship of k Values of Boranes Click here to View table |
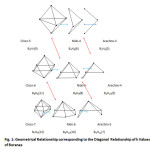 |
Fig1: Geometrical Relationship corresponding to the Diagonal Relationship of k Values of Boranes Click here to View Figure |
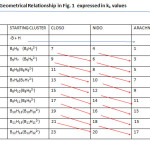 |
Table4: Geometrical Relationship in Fig. 1 expressed in k1 values Click here to View table |
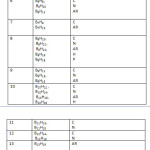 |
Table 5 Click here to View table |
C = CLOSO, N = NIDO, AR = ARACHNO, H = HYPO, A = CLASS A, P = CLASS P,
Q = CLASS Q, R = CLASS R
Table 1Extended left wise
| G | F | E | D | C0 | |
| n | BnHn-10 | BnHn-8 | BnHn-6 | BnHn-4 | BnHn-2 |
| 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | B2 |
| 3 | 0 | 0 | 0 | 0 | B3H |
| 4 | 0 | 0 | 0 | B4 | B4H2 |
| 5 | 0 | 0 | 0 | B5H | B5H3 |
| 6 | 0 | 0 | B6 | B6H2 | B6H4 |
| 7 | 0 | 0 | B7H | B7H3 | B7H5 |
| 8 | 0 | B8 | B8H2 | B8H4 | B8H6 |
| 9 | 0 | B9H | B9H3 | B9H5 | B9H7 |
| 10 | B10 | B10H2 | B10H4 | B10H6 | B10H8 |
| 11 | B11H | B11H3 | B11H5 | B11H7 | B11H9 |
| 12 | B12H2 | B12H4 | B12H6 | B12H8 | B12H10 |
| 13 | B13H3 | B13H5 | B13H7 | B13H9 | B13H11 |
| 14 | B14H4 | B14H6 | B14H8 | B14H10 | B14H12 |
| 15 | B15H5 | B15H7 | B15H9 | B15H11 | B15H13 |
| 16 | B16H6 | B16H8 | B16H10 | B16H12 | B16H14 |
| 17 | B17H7 | B17H9 | B17H11 | B17H13 | B17H15 |
| 18 | B18H8 | B18H10 | B18H12 | B18H14 | B18H16 |
| 19 | B19H9 | B19H11 | B19H13 | B19H15 | B19H17 |
| 20 | B20H10 | B20H12 | B20H14 | B20H16 | B20H18 |
Table 1 has been extended on the left to cover the boron families headed by symbols C0, D, E, and G. The whole Table 1 covers the series BnHn-10 to BnHn+14.
CONCLUSION
Borane series tend to occur in families just like hydrocarbons. The driving force for cluster formation is probably due to boranes attempting to obey the octet rule. A given borane that obeys the octet rule may be classified to belong to a particle family using the table. Both the known boranes and the theoretically established as being stable appear to fit into one of the families. Just as the elements of the periodic table follow a simple arithmetical progression, the boranes across the row horizontally (families- similar to the period in the periodic table)) and vertically( within the family members- similar to a group within the periodic table) as well as diagonally (similar to the diagonal relationship of elements in the periodic table) follow simple arithmetical progression. The k1 values are useful in organizing the boron families and their members
REFERENCES
- Kiremire, E. M. R.Oriental J. Chem.2007,32(2), 809-812.
- Kiremire, E. M. R.;Kiremire, E. B. B. Materials Science Research India2007,4(1), 09-18.
- Shaik, S.;Danovich, D.; Wu, W.; Su, P.; Rzepa, S.; Hiberty, P. C. Nature2012 Chemistry, 4, 195-200.
- Su, P.; Wu, J.; Gu, J.; Wu, W.; Shaik, S.; Hiberty, P. C. J. Chem. Theor. Comput. 2011,7, 121-130.
- Fox, M. A.; Wade, K. Pure Appl. Chem.2003, 75(9), 1315-1323.
- Yao, Y.; Hoffman, R. J. Am. Chem. Soc.,2011,133, 21002-21009.
- Böyükata, M.;özdoğana, C.; GÜvenç, Z. B.Romanian J. of Information Science and Technology200811(1), 59-70.

This work is licensed under a Creative Commons Attribution 4.0 International License.









