FT-IR Application for the Detection of Pistachio Oil Adulteration
Ali Sheibani*, Naser Ghotbaddini Bahraman and Fatemeh Sadeghi
Department of Chemistry, Yazd Branch, Islamic Azad University, Yazd, Iran
DOI : http://dx.doi.org/10.13005/ojc/300335
Article Received on :
Article Accepted on :
Article Published : 26 Aug 2014
In this work, fourier transform infrared spectroscopy (FT-IR) is used to identify and detection the adulteration of pistachio oil with cheap edible oils of corn, sunflower and soybean. For this purpose, pistachio oil was blended with cheap oils at concentration level of 10 to 60% (w/w). Then, FT-IR spectra of pure and adulterated pistachio oil samples were obtained. The fingerprints region was found to be useful in investigation of the adulteration of pistachio oil. At this region, the absorbance peaks of FT-IR decreased by increasing the adulterant amount with a linear relation that can be applied for the quality and quantity purposes. The obtained results showed that the proposed method can be considered and used as an alternative method in the detection and semi-quantization of adulteration in pistachio oil.
KEYWORDS:Pistachio oil; Adulteration; FT-IR
Download this article as:| Copy the following to cite this article: Sheibani A, Bahraman N. G, Sadeghi F. FT-IR Application for the Detection of Pistachio Oil Adulteration. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Sheibani A, Bahraman N. G, Sadeghi F. FT-IR Application for the Detection of Pistachio Oil Adulteration. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4355 |
INTRODUCTION
Analysis and control of the quality of edible oils is an important subject in most of the countries. Oils are complex mixtures containing a wide range of compounds such as di and tri-acylglycerrols, free fatty acids, phospholipids and other components. The adulteration of high cost oils with cheaper oils is a serious matter because there is a great price difference among them and also may cause harm to the health of consumers1-3. Among, numerous articles have been reported to detection or determine the adulteration olive oil with various seed oils4, 5.
Pistachio oil is also an expensive and important product that has a good market and can increase the added value of pistachio cultivation. Pistachio is one of the most significant oil seeds from the family of Anacadicea. It is composed of a kernel and a husk. The husk has a hard layer named shell and a softer layer called hull. The kernel has a green skin with a sweet taste and occupies different portions of the kernel in various types of pistachio. Compared to other nut oils, pistachio oil has a particularly strong flavor. Pistachio oil is rich in unsaturated fatty acids and vitamin E and also is useful for health. It contains 12.7% saturated fats, 53.8% monosaturated fats, 32.7% linoleic acid and 0.8% omega-3 fatty acid. Pistachio oil is used as a table oil to add flavor to foods. It is also used in skin care products6-8. Therefore, adulteration of it involving the replacement with lower grade and cheaper oils can be performed.
Several techniques were proposed to control and detect the adulteration of edible oils. HPLC, GC, UV, NMR, Chemiluminescence and Fiber optic sensor have been widely used for this purpose9-14. Fourier transform infrared spectroscopy (FT-IR) is a technique used in the food product adulteration area or quality control. It is a rapid, non-destructive technique with short time for the sample analysis. FT-IR can be also used for quantitative analysis purposes. The intensity of bands in the spectrum is proportional to the concentration of the studied compounds15-18.
In present study, FT-IR is developed for the detection of pistachio oil adulteration with other edible oils including: corn, sunflower and soybean. Based on results and also because of simplicity and low cost, FT-IR can be applied as an alternative analytical method for the pistachio oil adulteration.
EXPERIMENTAL
Materials and Methods
Pistachio samples were obtained from Rafsanjan area of Iran. The pistachio kernel was ground and its oil extracted using Soxhlet procedure for 3 hr with n-hexane as the extraction solvent. Corn, sunflower and soybean oils were purchased from local supermarkets. Chemicals and solvents used in this work purchesd from Merck (Darmstadt, Germany).
FT-IR spectra of the oil samples were recorded on a Bruker Tensor-27 spectrometer. Different mixture samples (w/w%) of various kinds of vegetable oils with pistachio oil were prepared. 5 µL of pure and blended pistachio oils was deposited and spread as homogenously and one layer between two plates of NaCl crystal. For obtain the good results, this step is important. The prepared samples spectra (FT-IR) were recorded within 4000 to 400 cm-1. The peaks height in the wavenumbers of 1094, 1116 and 1164 cm-1 were considered and used in the adulteration study of pistachio oil. The equations for study of the pistachio oil adulteration in blended samples were obtained by least squares linear regression.
RESULTS AND DISCUSSION
The first part of this study involved structurally differentiating between pistachio oil and the mixture of it with mentioned cheap oils and then detecting and determining the adulteration of pistachio oil. For this purpose, the spectra of pure pistachio oil were obtained and compared with the spectra of cheap oils and also pistachio oil blended to them.
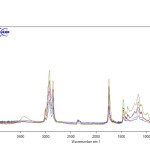 |
Fig1: FT-IR spectra of pistachio oil (top) mixed with different ratios (25, 50, 75 and 100) of corn oil. Click here to View Figure |
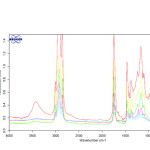 |
Fig2: FT-IR spectra of pistachio oil (top) mixed with different ratios (25, 50, 75 and 100) of sunflower oil. Click here to View Figure |
FT-IR spectra of the edible oils show that there is a notable difference in the spectral region between 1300-1000 cm-1,so that the oil composition affects the position of band and its intensity5. This is found to be very useful in detecting the edible oil adulteration. Figs. 1-3 show the FT-IR spectra of the pure and mixed pistachio oil with different ratios of corn, and sunflower soybean oils. The intensity of the absorption peak at 1094, 1116 and 1164 cm-1 decreases by increasing the amount of adulterant oils. Based on this, the percentage of the added edible oil to pistachio oil was determined. Table 1 shows the equations of change in the peak height in the mentioned wavenumbers with various percentages of cheap edible oils. Correlation coefficients have also been reported that shown a good linear relation between them. The lowest correlation coefficient (0.9320 at 1116 cm-1) is observed for adulteration with sunflower oil while the best results (0.9742 at 1164 cm-1) are seen for corn oil.
 |
Fig3: FT-IR spectra of pistachio oil (top) mixed with different ratios (25, 50, 75 and 100) of soybean oil. Click here to View Figure |
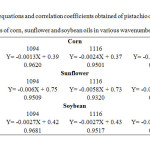 |
Table1: The equations and correlation coefficients obtained of pistachio oil mixed with different ratios of corn, sunflower and soybean oils in various wavenumbers (cm-1). Click here to View table |
In order to evaluation of the proposed method, different amounts of the cheap edible oil were added to pistachio oil and the adulteration percentage was calculated. The recovery results were given at Table 2, showed a good agreement between the added and found amounts of cheap oils for the adulteration pistachio oil study. According to results at this Table, the proposed method can successfully be applied to the detection and semi-quantitative assignment of pistachio oil adulteration. It can also perform to the development in food control and analysis.
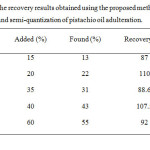 |
Table2: The recovery results obtained using the proposed method in detection and semi-quantization of pistachio oil adulteration. Click here to View table |
CONCLUSION
In summary, the present work developed the FT-IR application for the monitoring and detecting of the adulteration of pistachio oil with the lower priced edible oils. The absorbance changes with increasing the amount of adulterant oil were used for the detecting of adulteration. The results prove the validity and capability of the proposed method in detection of the pistachio oil adulteration. Adulteration as low as 10% could be detected rapidly using FT-IR. The study leads to the conclusion that the proposed method can be potentially considered as an alternative procedure in the quality control of pistachio oil and other expensive oils.
ACKNOLEDGMENT
The authors would like to acknowledge the IAU. Yazd Branch, for financial support of this work.
REFERENCES
- Rohman, A.; Cheman, Y. B. J. Food Lipids 2009, 16, 618-628.
- Obeidat, S. M.; Khanfar, M. S.; Obedat,W. M. Aust. J. Basic Appl. Sci. 2009, 3, 2048-2053.
- Özdemir, D.; Özturk, B. J. Food Drug Anal. 2007, 15, 40-47.
- Cserháti, T.; Forgács, E.; Deyl, Z.; Miksik, I. Biomed. Chromatogr. 2005, 19, 183-190.
- Allam, M. A.; Hamed, S. F. J. Appl. Sci. Res. 2007, 3, 102-108.
- Sheibani, A.; Ghaziaskar, H. S. LWT- Food Sci. Technol. 2008, 41, 1472-1477.
- www. hsph. harvard. edu/nutritionsource.
- www. en. wikipedia. or/wiki/pistachio oil.
- Cordela, C.; Moussa, I.; Martel, A. C.; Sbirrazzuoli, N.; Lizzani-Cuvelier, L. J. Agric Food Chem. 2002, 50, 1751-1764.
- Andrikopoulos, N. K.; Giannakis, I.; Tsamtzis, V. J. Chrom. Sci. 2001, 39, 137-145.
- Mavromoustakos, T.; Zervou, M.; Bonas, Z. G.; Coulouris, A.; Petrakis, P. J. Am. Oil Chem. Soc. 2000, 77, 405-411.
- Papadopoulos, K.; Triantis, T.; Tzikis, C. H.; Nikokavoura, A.; Dimotikali, D. Anal. Chim. Acta 2002, 464, 135-140.
- Libish, T. M.; Linesh, J.; Bobby, M. C.; Biswas, P.; Bandyopadhyay, S.; Dasgupta, K.; Radhakrishnan, P. Optoelectron Adv. Mater. 2011, 5, 68-72.
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A., Tegou, E. Anal. Chim. Acta 2006, 573, 459-465.
- Tay, A.; Singh, R. K.; Krishnan, S. S.; Gore, J. P. LWT-Food Sci. Technol. 2002, 35, 99-103.
- Baeten, V.; Aparicio, R. Biotechnol Agron. Soc. Environ. 2000, 4, 196-203.
- Zhang, Q.; Liu, C.; Sun, Z.; Hu, X.; Shen, Q.; Wu, J. Food Chem. 2012, 132, 1607-1613.

This work is licensed under a Creative Commons Attribution 4.0 International License.









