Benzoin Condensation and Stetter Reaction Catalysed by N,N-Dimethylbenzimidazolium Iodide in [Bmim][OH]
Baramee Phungpis, Viwat Hahnvajanawong* and Parinya Theramongkol
Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand
DOI : http://dx.doi.org/10.13005/ojc/300303
Article Received on :
Article Accepted on :
Article Published : 12 Sep 2014
N,N-dimethylbenzimidazolium iodide catalysed benzoin condensation and Stetter reaction in 1-butyl-3-methylimidazolium hydroxide [bmim][OH] which acts as a basic catalyst as well as a solvent for the reactions are described. The recycled reaction media containing benzimidazolium salt can be reused for several times without significant loss of efficiency.
KEYWORDS:Benzoin condensation; Stetter reaction; N; N-dimethylbenzimidazolium iodide; [bmim][OH]
Download this article as:| Copy the following to cite this article: Phungpis B, Hahnvajanawong V, Theramongkol P. Benzoin Condensation and Stetter Reaction Catalysed by N,N-Dimethylbenzimidazolium Iodide in [Bmim][OH]. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Phungpis B, Hahnvajanawong V, Theramongkol P. Benzoin Condensation and Stetter Reaction Catalysed by N,N-Dimethylbenzimidazolium Iodide in [Bmim][OH]. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4578 |
INTRODUCTION
Recently a basic ionic liquid [bmim][OH] has been extensively used as a basic catalyst and reaction medium for various organic reactions such as Michael addition1, Markovnikov addition2, Knoevenagel condensation3 and Erlenmeyer Plochl reaction4.
In our previous reports5,6 benzoin condensation and Stetter reaction catalysed by N,N-dimethylbenzimidazolium iodide and NaOH were successfully performed in a neutral ionic liquid [bmim][PF6]. To the best of our knowledge, there is no report of the two reactions conducted in the ionic liquid [bmim][OH]. Herein, as part of our continuing interest in carrying out reaction in ionic liquid as an environmentally benign solvent, we reported the use of [bmim][OH] as dual basic catalyst and reaction medium for the N,N-dimethylbenzimidazolium iodide catalysed benzoin condensation and Stetter reaction.
EXPERIMENTAL
Chemicals were perchased from Fluka, Aldrich and Merck chemical company and used without further purification. Melting points were determined on a Sanyo Gallenkamp melting point apparatus and compared with those of known samples. IR spectra were recorded on a Perkin Elmer Spectrum One FT-IR Spectrometer. 1H and 13C NMR spectra were obtained using a VARIAN MERCURY plus (400 MHz FT NMR).
N,N-Dimethylbenzimidazolium iodide was prepared following our previously reported reference7.
General procedure for benzoin condensation catalysed by N,N-dimethylbenzimidazolium iodide (2) in [bmim][OH]
To a stirred solution of N,N-dimethylbenzimidazolium iodide (2) (0.055 g, 0.20 mmol) in [bmim][OH] (2 ml) was added an aromatic aldehyde (1.00 mmol) at room temperature. The temperature was raised to 80 °C and the resulting mixture was stirred for 4–8 h. After completion of the reaction, as determined by TLC (100% CH2Cl2), the reaction mixture was extracted with EtOAc (3´80 ml). The organic layers were combined and washed with water and dried with Na2SO4 and concentrated under reduced pressure. The residue was purified using preparative thin layer chromatography on silica gel with CH2Cl2 as eluant.
The purified compounds (3a-d and 4a-d) with their physical data are listed below.
Benzoin (3a). White crystals; mp 134–136 °C (lit.8 134–136 °C). FTIR (KBr, n, cm-1) : 3414 (O-H stretching), 3057, 3021 (aromatic C-H stretching), 2934 (aliphatic C-H stretching), 1674 (C=O stretching), 1595(aromatic C=C stretching), 1266 (C-O stretching). 1H NMR (CDCl3, 400 MHz) d: 7.91 (2H, d, J = 7.2 Hz, 2- and 6-H), 7.52 (1H, t, J = 7.2 Hz, 4-H), 7.39 (2H, t, J = 7.6 Hz, 3- and 5-H), 7.26–7.35 (5H, m, ArH), 5.95 (1H, s, CH), 4.54 (1H, br s, OH); 13C NMR (CDCl3) d: 76.2, 127.7, 128.6, 128.7, 129.1, 133.5, 133.9, 139.0, 198.9.
4,4/-Dimethylbenzoin (3b). White crystals; mp 73–75 °C (lit.9 75–76 °C). FTIR (KBr, n, cm-1): 3458 (O-H stretching), 3030 (aromatic C-H stretching), 2921 (aliphatic C-H stretching), 1673 (C=O stretching), 1607 (aromatic C=C stretching), 1277 (C-O stretching). 1H NMR d 7.81 (2H, d, J = 8.4 Hz, 2- and 6-H), 7.21 (2H, d, J = 8.4 Hz, 3- and 5-H), 7.18 (2H, d, J = 8.0 Hz, 2¢-H and 6¢-H), 7.11 (2H, d, J = 8.0 Hz, 3¢- and 5¢-H), 5.89 (1H, s, CH), 2.35 (3H, s, Ar-CH3), 2.28 (3H, s, Ar-CH3); 13C NMR (CDCl3) d: 21.1, 21.7, 75.8, 127.6, 129.3, 129.4, 129.8, 131.0, 136.4, 138.3, 144.9, 198.5.
4,4/-Dichlorobenzoin (3c). White crystals; mp 87–88 °C (lit.8 88 °C). FTIR (KBr, n, cm-1): 3425 (O-H stretching), 3072 (aromatic C-H stretching), 2929 (aliphatic C-H stretching), 1674 (C=O stretching), 1590 (aromatic C=C stretching), 1252 (C-O stretching), 812 (C-Cl stretching). 1H NMR (CDCl3, 400 MHz) d: 7.75 (2H, d, J = 8.4 Hz, 2- and 6-H), 7.32 (2H, d, J = 8.4 Hz, 3- and 5-H), 7.24 (2H, d, J = 8.4 Hz, 3/– and 5/–H), 7.18 (2H, d, J = 8.4 Hz, 2/– and 6/–H), 5.81 (1H, s, CH); 13C NMR (CDCl3) d: 75.5, 129.0, 129.2, 129.4, 130.4, 131.5, 134.8, 137.1, 140.7, 197.4.
4,4/-Difluorobenzoin (3d). White crystals; mp 83–84 °C (lit.11 81-82 °C). FTIR (KBr, n, cm-1): 3451 (O-H stretching), 3075(aromatic C-H stretching), 2928 (aliphatic C-H stretching), 1677 (C=O stretching), 1597 (aromatic C=C stretching), 1232 (C-O stretching), 1156 (C-F stretching). 1H NMR (CDCl3, 400 MHz) d: 7.93 (2H, dd, J = 8.8 Hz and 5.2 Hz, 2- and 6-H), 7.30 (2H, dd, J = 8.8 Hz and 5.2 Hz, 2¢¢-H and 6¢¢-H), 7.08 (2H, t, J = 8.8 Hz, 3¢-H and 5¢-H), 7.02 (2H, t, J = 8.4 Hz, 3¢¢- and 5¢¢-H), 5.90 (1H, s, CH), 4.54 (1H, br s, OH) ; 13C NMR d 75.3, 116.0, 116.1, 116.2, 116.3, 129.4, 129.5, 129.6, 129.7, 131.8, 131.9, 134.7, 134.8, 161.5, 164.0, 164.8, 167.3, 197.2.
O-Benzoylbenzoin (4a). Yellow crystals; mp 123–125 °C (lit.8 125 °C). FTIR (KBr, n, cm-1): 3064, 3034 (aromatic C-H stretching), 2959 (aliphatic C-H stretching), 1717, 1695 (C=O stretching), 1599 (aromatic C=C stretching), 1278, 1108 (C-O stretching). 1H NMR d 8.13 (2H, d, J = 7.6 Hz, 2¢¢- and 6¢¢-H), 8.01 (2H, d, J = 8.0 Hz, 2- and 6-H), 7.39-7.59 (11H, m, ArH), 7.10 (1H, s, CH); 13C NMR d 77.9, 128.4, 128.6, 128.8, 129.1, 129.3, 129.4, 130.0, 133.3, 133.4, 133.8, 134.8, 166.0, 193.7.
O-(4-Methylbenzoyl)-4,4/-dimethylbenzoin (4b). Yellow liquid. FTIR (neat, n, cm-1): 3058, 3032 (aromatic C-H stretching), 2957 (aliphatic C-H stretching), 1707, 1690 (C=O stretching), 1610 (aromatic C=C stretching), 1283, 1114 (C-O stretching). 1H NMR (CDCl3), 400 MHz) d: 8.00 (2H, d, J = 8.0 Hz, 2//– and 6//–H), 7.90 (2H, d, J = 8.0 Hz, 2- and 6-H), 7.45 (2H, d, J = 8.0 Hz, 3- and 5-H), 7.18–7.23 (6H, m, ArH) 7.04 (1H, s, CH), 2.40 (3H, s, Ar-CH3), 2.35 (3H, s, Ar-CH3), 2.32 (3H, s, Ar-CH3); 13C NMR (CDCl3) d: 21.2, 21.6, 21.7, 77.6, 126.8, 128.6, 129.0, 129.1, 129.3, 129.8, 130.0, 131.2, 132.2, 139.2, 144.0, 144.3, 166.1, 193.4.
O-(4-Chlorobenzoyl)-4,4/-dichlorobenzoin (4c). Yellow crystals; mp 125–127 °C (lit.10 127–128 °C). FTIR (KBr, n, cm-1): 3095 (aromatic C-H stretching), 2925 (aliphatic C-H stretching), 1722, 1698 (C=O stretching), 1592 (aromatic C=C stretching), 1249, 1090 (C-O stretching), 757 (C-Cl stretching). 1H NMR (CDCl3, 400 MHz) d: 7.95 (2H, d, J = 8.8 Hz, 2¢¢- and 6¢¢-H), 7.82 (2H, d, J = 8.4 Hz, 2- and 6-H), 7.29-7.41 (8H, m, ArH), 6.91 (1H, s, CH); 13C NMR d 77.1, 127.5, 128.9, 129.2, 129.6, 129.9, 130.1, 131.3, 131.7, 132.7, 135.8, 140.1, 140.4, 165.0, 192.1.
O-(4-Fluorobenzoyl)-4,4/-difluorobenzoin (4d). Yellow liquid; IR (KBr, n, cm-1): 3082 (aromatic C-H stretching), 2926 (aliphatic C-H stretching), 1724, 1687 (C=O stretching), 1601, 1508 (aromatic C=C stretching), 1285, 1154 (C-O stretching), 1106 (C-F stretching). 1H NMR (CDCl3, 400 MHz) d: 8.12 (2H, dd, J = 8.8 and 5.2 Hz, 2¢¢- and 6¢¢-H), 8.01 (2H, dd, J = 8.8 and 5.2 Hz, 2- and 6-H), 7.54 (2H, dd, J = 8.8 and 5.2 Hz, 2¢- and 6¢-H), 7.08-7.14 (6H, m, ArH), 7.02 (1H, s, CH); 13C NMR d 76.7, 115.6, 115.8, 116.0, 116.2, 116.3, 116.5, 125.4, 128.5, 129.4, 130.2, 130.6, 130.7, 130.9, 131.0, 131.5, 131.6, 132.6, 132.7, 162.1, 164.6, 164.7, 164.9, 166.0, 167.3, 167.4, 192.0.
General procedure for Stetter reaction between either acryonitrile (5) or ethyl acrylate (7) and aromatic aldehydes catalysed by N,N-dimethylbenzimidazolium iodide (2) in [bmim][OH]
To a stirred solution of N,N-dimethylbenzimidazolium iodide (2) (0.055 g, 0.20 mmol) in [bmim][OH] (2 ml) was added either acryonitrile (5) or ethyl acrylate (7) (2.00 mmol) and an aromatic aldehyde (1.00 mmol) at room temperature. The temperature was raised to 80 °C and the resulting mixture was allowed to stir for 14–17 h. After completion of the reaction, as determined by TLC (100% CH2Cl2), the reaction mixture was extracted with ethyl acetate (3´80 ml). The organic layers were combined and washed with water and dried with Na2SO4 and concentrated under reduced pressure. The residue was purified using preparative thin layer chromatography on silica gel with CH2Cl2 as eluant.
The purified compounds (6a-c, 8a-c, 9d and 10d) with their physical data are listed below.
4-Phenyl-4-oxobutanenitrile (6a). White crystals; mp 75-77 oC (lit.12 76 oC). FTIR (KBr, n, cm-1): 3067 (aromatic C-H stretching), 2923, 2882 (aliphatic C-H stretching), 2252 (C≡N stretching), 1673 (C=O stretching), 1604 (aromatic C=C stretching). 1H NMR d 7.96 (2H, d, J = 7.2 Hz, 2¢- and 6¢-H), 7.62 (1H, t, J = 7.6 Hz, 4¢-H), 7.50 (2H, t, J = 7.6 Hz, 3¢- and 5¢-H), 3.39 (2H, t, J = 7.2 Hz, CH2CH2CN), 2.78 (2H, t, J = 7.2 Hz, CH2CH2CN); 13C NMR d 11.8, 34.2, 119.2, 128.0, 128.8, 133.9, 135.6, 195.3.
4-(4/-Tolyl)-4-oxobutanenitrile (6b). White crystals; mp 75-77 oC (lit.13 75 oC). FTIR (KBr, n, cm-1): 3040 (aromatic C-H stretching), 2924, 2853 (aliphatic C-H stretching), 2251(C≡N stretching), 1673 (C=O stretching), 1606 (aromatic C=C stretching). 1H NMR d 7.86 (2H, d, J = 8.0 Hz, 2¢- and 6¢-H), 7.29 (2H, d, J = 8.0 Hz, 3¢- and 5¢-H), 3.37 (2H, t, J = 7.2 Hz, CH2CH2CN), 2.77 (2H, t, J = 7.2 Hz, CH2CH2CN), 2.43 (3H, s, Ar-CH3); 13C NMR d 11.8, 29.7, 34.1, 119.2, 128.1, 129.5, 133.2, 144.9, 201.4.
4-(4/-Chlorophenyl)-4-oxobutanenitrile (6c). White crystals; mp 70-72 oC (lit.14 72-73 oC). FTIR (KBr, n, cm-1): 3091, 3060 (aromatic C-H stretching), 2924 (aliphatic C-H stretching), 2250 (C≡N stretching), 1675 (C=O stretching), 1590 (aromatic C=C stretching), 772 (C-Cl stretching). 1H NMR d 7.89 (2H, d, J = 8.4 Hz, 2¢- and 6¢-H), 7.47 (2H, d, J = 8.4 Hz, 3¢- and 5¢-H), 3.35 (2H, t, J = 7.2 Hz, CH2CH2CN), 2.77 (2H, t, J = 7.2 Hz, CH2CH2CN); 13C NMR d 11.7, 34.2, 119.0, 129.2, 129.4, 133.9, 140.5, 194.1.
Ethyl 4-phenyl -4-oxobutanoate (8a). Yellow liquid. FTIR (neat, n, cm-1): 3063 (aromatic C-H stretching), 2983, 2933 (aliphatic C-H stretching), 1733, 1687(C=O stretching), 1597 (aromatic C=C stretching), 1219, 1031 (C-O stretching). 1H NMR d 7.97 (2H, d, J = 7.2 Hz, 2¢- and 6¢-H), 7.55 (1H, t, J = 7.2 Hz, 4¢-H), 7.45 (2H, t, J = 7.2 Hz, 3¢- and 5¢-H), 4.15 (2H, q, J = 7.2 Hz, OCH2CH3), 3.30 (2H, t, J = 6.8 Hz, CH2CH2CO2CH2CH3), 2.75 (2H, t, J = 6.8 Hz, CH2CH2CO2CH2CH3 ), 1.25 (3H, t, J = 7.2 Hz, CO2CH2CH3); 13C NMR d 14.2, 28.3, 33.4, 60.6, 128.0, 128.6, 133.2, 136.6, 172.9, 198.1.
4-(4/-Tolyl)-4-oxobutanoate (8b). White crystals; mp 64-65 oC; IR (KBr, n, cm-1): 3091 (aromatic C-H stretching), 2981, 2930 (aliphatic C-H stretching), 1732, 1671 (C=O stretching), 1589 (aromatic C=C stretching), 1174, 1089 (C-O stretching). 1H NMR d 7.85 (2H, d, J = 8.4 Hz, 2¢- and 6¢-H), 7.37 (2H, d, J = 8.4 Hz, 3¢- and 5¢-H), 4.09 (2H, q, J = 7.2 Hz, CO2CH2CH3), 3.21 (2H, t, J = 6.4 Hz, CH2CH2CO2CH2CH3), 2.69 (2H, t, J = 6.4 Hz, CH2CH2CO2CH2CH3 ), 2.10 (3H , s, Ar-CH3), 1.20 (3H, t, J = 7.2 Hz, CO2CH2CH3); 13C NMR d 14.2, 28.2, 30.9, 33.0, 60.7, 128.9, 129.4, 134.9, 139.6, 172.7, 196.9.
4-(4/-Dichlorophenyl)-4-oxobutanoate (8c). White crystals; mp 56-58 oC (lit.16 58-59 oC). FTIR (KBr, n, cm-1): 3003 (aromatic C-H stretching), 2950, 2846 (aliphatic C-H stretching), 1749, 1659 (C=O stretching), 1598 (aromatic C=C stretching), 1270, 1105 (C-O stretching), 775 (C-Cl stretching). 1H NMR d 7.85 (2H, d, J = 8.8 Hz, 2¢- and 6¢-H), 7.37 (2H, d, J = 8.8 Hz, 3¢- and 5¢-H), 4.09 (2H, q, J = 7.2 Hz, CO2CH2CH3), 3.20 (2H, t, J = 6.8 Hz, CH2CH2CO2CH2CH3), 2.68 (2H, t, J = 6.8 Hz, CH2CH2CO2CH2CH3), 1.20 (3H, t, J = 7.2 Hz, CO2CH2CH3); 13C NMR d 14.2, 28.2, 33.3, 60.7, 128.9, 129.4, 134.9, 139.6, 172.7, 196.9.
4,5-Di(4-fluoro)-4-hydroxy-5-oxopentane (9d). Colourless liquid. FTIR (neat, n, cm-1): 3428 (O-H stretching), 3030 (aromatic C-H stretching), 2925, 2855 (aliphatic C-H stretching), 2249 (C≡N stretching), 1677 (C=O stretching), 1606 (aromatic C=C stretching), 1255 (C-O stretching), 1183 (C-F stretching). 1H NMR d 7.79 (2H, dd, J = 8.4 and 5.6 Hz, 2- and 6-H), 7.41 (2H, dd, J = 8.4 and 5.2 Hz, 2¢- and 6¢-H), 7.11 (2H, t, J = 8.4 Hz, 3- and 5-H), 7.02 (2H, t, J = 8.8 Hz, 3¢- and 5¢-H), 2.56-2.69 (2H, m, CH2CH2CN), 2.38-2.46 (1H, m, HCHCH2CN), 2.19-2.27 (1H, m, HCHCH2CN); 13C NMR d 11.8, 34.9, 80.6, 115.7, 116.0, 116.2, 116.4, 118.1, 119.2, 127.2, 127.3, 129.5, 132.9, 133.0, 135.8, 159.9, 161.5, 163.9, 167.0 198.0.
4,4/-Difluorobenzil (10d). White crystals; mp 119-121 oC (lit.15 121 oC). FTIR (KBr, n, cm-1): 3076 (aromatic C-H stretching) , 1664 (C=O stretching), 1598 (aromatic C=C stretching), 1157 (C-F stretching). 1H NMR δ 8.02 (4H, dd, J = 8.8 Hz and 5.2 Hz, 2-H, 6-H, 2¢-H and 6¢-H), 7.20 (4H, t, J = 8.4 Hz, 5-H, 3-H, 5¢-H and 3¢-H); 13C NMR d 116.4, 116.6, 128.8, 129.4, 132.8, 132.9, 165.6, 168.2, 192.2.
RESULTS AND DISCUSSION
Benzoin condensation and Stetter reaction were carried out under similar conditions as previously reported5,6 excepted for the using of 20 mol% of N,N-dimethylbenzimidazolium iodide (2) in basic ionic liquid [bmim][OH] at 80 °C. As shown in Scheme I, benzoin condensation of aromatic aldehydes 1a-d proceeded very efficiently in [bmim][OH]. Similar to our previous results using [bmim][PF6]5, aroins 3a-d underwent further transformation to the corresponding aroylaroins 4a-d by the mechanism reported by Miyashita et al.10.
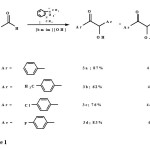 |
Scheme 1Click here to View Scheme |
Stetter reaction between acrylonitrile (5) and aromatic aldehydes 1a-c in [bmim][OH] gave the corresponding adducts 4-aryl-4-oxobutanenitriles 6a-c, in satisfactory yields as illustrated in Scheme 2. Unlike our previous reactions in [bmim][PF6]5, benzoin condensation was the only side reaction as evidenced by isolation of the corresponding aroins 3a-c as minor products.
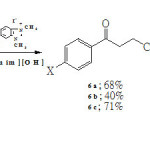 |
Scheme 2 Click here to View Scheme |
Similar results were also obtained upon carrying out Stetter reaction using a less electrophilic double bond, i.e., ethyl acrylate (7). Benzoin condensation, however, became the more competitive side reaction. While the percent yields of ethyl 4-aryl-4-oxobutanoates 8a-c decreased, percent yields of aroins 3a-c increased significantly as illustrated in Scheme 3.
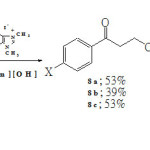 |
Scheme 3 Click here to View Scheme |
Stetter reaction between either acrylonitrile (5) or ethyl acrylate (7) and 4-fluorobenzaldehyde (1d) on the other hand gave different results. As shown in Scheme 4, treatment of acrylonitrile (5) with 4-fluorobenzaldehyde (1d) gave 5-oxonitrile 9d and aril 10d as the only isolated products. Contrastingly, when ethyl acrylate (7) was employed as electrophilic double bond the Stetter adduct could no longer be detected. Only aroin 3d and aril 10d were the two isolated products.
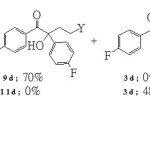 |
Scheme 4 Click here to View Scheme |
Formation of 5-oxonitrile 9d was resulted from further reaction of 4-oxonitrile 6 (X = F) by the mechanism proposed in our previous report [6]. Further oxidation of aroin 3d by the mechanism suggested by our group7 was responsible for the formation of aril 10d. Treatment of ethyl acrylate (7) with 4-fluorobenzaldehyde (1d) gave none of the Stetter adduct because 4-fluorobenzaldehyde (1d) was a much better acyl anion receptor than ethyl acrylate (7), therefore, the cross-coupling reaction between ethyl acrylate (7) and 4-fluorobenzaldehyde (1d) could not efficiently proceed.
It was also found that the recycled reaction media from either benzoin condensation or Stetter reaction, containing benzimidazolium salt, can be reused in the same type of reaction for up to at least 3 cycles. The same product distributions without significant decreasing in their yields were obtained.
CONCLUSION
Benzoin condensation of aromatic aldehydes catalysed by N,N-dimethylbenzimidazolium iodide in [bmim][OH] proceeded very well with no additional hydroxide base. Aroylaroins were found as the only minor products. Stetter reaction between either acrylonitrile or ethyl acrylate and aromatic aldehydes catalysed by benzimidazolium salt also performed well in [bmim][OH], although benzoin condensation occurred competitively. No Stetter reaction was observed upon treatment of ethyl acrylate with 4-fluorobenzaldehyde.
ACKNOWLEDGEMENT
Financial support from the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Office of the Higher Education Commission, Ministry of Education is gratefully acknowledged.
REFERENCES
- Ranu, B. C.; Banerjee, S.; Jana, R. Tetrahedron 2007, 63, 776-782
- Xu, J.-M.; Lu, B.-K.; Wu, W.-B.; Qian, C.; Wu, Q.; Lin, X-F. J. Org. Chem. 2006, 71, 3991-3993
- Ranu, B. C.; Jana, R. Eur. J. Org. Chem. 2006, 3767–3770
- Patil, S. G.; Bagul, R. R.; Swami, M. S.; Hallale, S. N.; Kamble, V. M. J. Chem. Pharm. Res. 2011, 3, 457-463
- Hahnvajanawong, V.; Waengdongbung, W.; Piekkaew, S.; Phungpis, B.; Theramongkol, P. ScienceAsia 2013, 39, 50-55
- Hahnvajanawong, V.; Phungpis, B.; Theramongkol, P. ACGC Chem. Res. Comm. 2009, 23, 26-30
- Hahnvajanawong, V.; Tearavarich, R.; Theramongkol, P. ACGC Chem. Res. Comm. 2005, 18, 7-10
- Buckingham, J., Dictionary of Organic Compounds, 5th ed.; Chapman and Hall. New York, (1982)
- Breslow, R. J. Am. Chem. Soc. 1958, 80, 3719-3726
- Miyashita, A.; Matsuda, H.; Iijima, C.; Higashino, T. Chem. Pharm. Bull. 1990, 38, 1147-1152
- Demir, A. S.; Sesenoglu, O.; Eren, E.; Hosrik, B.; Pohl, M.; Janzen, E.; Kolter, D.; Feldmann, R.; Dunkelmann, P.; Muller, M. Adv. Synth. Catal. 2002, 344, 96-103
- Stetter, H.; Schreckenberg, M. Angew. Chem. Inernat. Edit. 1973, 12, 81
- Reutrakul, V.; Nimgirawath, S.; Panichanun, S,; Ratananukul, P. Chem. Lett, 1979, 399-400
- Herman, H., Schreckenberg, M., US Patent NO. 4014889, (1977)
- Pouchert, C.J., The Aldrich Library of NMR Spectra, Vol 2. Milwaukee, Wisconsin. 877, (1991)
- Stetter, H.; Kuhlmann, H. Org. React. 1991, 40, 434

This work is licensed under a Creative Commons Attribution 4.0 International License.









