Adsorption of Lead (II) from aqueous solutions onto activated carbon prepared from Algerian dates stones of Phoenix dactylifera.L (Ghars variety) by H3PO4 activation
N. Chaouch1, M. R. Ouahrani2 and S.E. Laouini2
1Unit of Formulation – Colloids and nano systems, Dynamics interactions and reactivity of systems (DIRES) laboratory, faculty of applied sciences, University of Ouargla, Ouargla 30000, Algeria
2Valorization and Technology of Saharian Resource laboratory, Faculty of Science and Technology, El Oued University, ElOued, Algeria
DOI : http://dx.doi.org/10.13005/ojc/300349
Article Received on :
Article Accepted on :
Article Published : 19 Sep 2014
Currently water pollution constitutes a great challenge, and activated carbon is a common adsorbent used to remove lead contaminants. Unfortunately, it is a non selective process. The main object of this study was the use of an activated carbon prepared from nuts of dates Algerian origin to remove this metal. The adsorption measurement of lead on activated carbon showed a real potential for removing this metal contaminants waste. The result showed also that the determination of lead remained dependent on some parameters such as pH, time contact, temperature and initial concentrations of metal. Adsorption data followed Freundlich model, they were better fitted by Langmuir isotherm as compared to Freundlich.
KEYWORDS:adsorption; activated carbon; lead removal; Langmuir model; Freundlich model
Download this article as:| Copy the following to cite this article: Chaouch N, Ouahrani M. R, Laouini S. E. Adsorption of Lead (II) from aqueous solutions onto activated carbon prepared from Algerian dates stones of Phoenix dactylifera.L (Ghars variety) by H3PO4 activation. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Chaouch N, Ouahrani M. R, Laouini S. E. Adsorption of Lead (II) from aqueous solutions onto activated carbon prepared from Algerian dates stones of Phoenix dactylifera.L (Ghars variety) by H3PO4 activation. Orient J Chem 2014;30(3). Available from: "http://www.orientjchem.org/?p=4824" |
INTRODUCTION
The removal of toxic heavy metals such as lead, cadmium, copper, nickel, mercury and zinc from aqueous environment has received considerable attention in recent years due to their toxicity which may cause damage to various systems of the human body. They also can be absorbed by marine animals and directly enter the human food chains, thus presenting a high health risk to consumers1,2,3,4,5.
Lead is found to be a toxic heavy metal in waste water generated by various industries such as metal plating, metal finishing and metal processing industries6.
The constant exposure to Pb(II) causes edema, learning and behavioral difficulties in children, damage to organs including the liver, kidneys and heart and disturbances to the immune system. The adverse effects of lead have been noted at concentration between 0,01 and 5 mg/l. therefore the concentration of Pb(II) in aqueous solution should be reduced to attain stringent rules put forwarded by various government and international organizations6,7,8.
Several methods are commonly used for the treatment of heavy metals, such as adsorption, chemical precipitation, coagulation, flocculation, extraction and reverse osmosis9,10.11,12,13,14. Most of these process need high capital cost15. They have great disadvantage by operating in a succession of steps of heterogeneous reactions, or distribution of substances between different phases which usually requires a lengthy operating period. Moreover, the final metal recovery requires additional treatments, which complicate the process16.
Adsorption has been shown to be an economical alternative for removing trace of metals from water. Among the numerous materials applied in adsorption processes, activated carbons, primarily due to their low cost and high adsorption ability, are still the most widely used materials for adsorption of impurities17,18,19.
Basically, there are two different processes for the preparation of activated carbon: physical activation and chemical activation. Physical activation involves carbonization of a carbonaceous material followed by the activation of the resulting char at a temperature between 1073 and 1373 °K in the presence of suitable oxidizing gases such as carbon dioxide or steam. In chemical activation, the precursor is mixed with a chemical agent and then pyrolyzed between 673 and 873 °K in the absence of air5.
Among the numerous dehydrating agents, phosphoric acid in particular is the widely used chemical agent in the preparation of activated carbons20.
The aim of this work was to evaluate the feasibility of using activated carbon prepared from an algerian dates stones of Phoenix dactylifera.L (Ghars variety) cultivated in Ouargla region situated in the south of Algeria for the removal of Pb(II) from aqueous solution. The influence of experimental conditions such as pH, contact time, initial metal concentration and solution temperature were studies.
METHODS AND EXPERIMENTAL
The tests stock solutions were prepared by dissolving lead nitrate Pb(NO3)2 in distilled water. The pH of the test solutions was adjusted using hydrochloric acid (0.1M) or sodium hydroxide (0.1M)9.
Materials
The pH measurements were made using a pH meter (Hanna 4221).The lead concentrations in the sample were determined using atomic adsorption spectrophotometer (Thermoelectron corporation). During adsorption tests, a shaker (flask) was used. A furnace (Select-Horn) was utilized in the preparation of Activated carbon. The other equipments used in the characterization of adsorbent are being listed in the next section.
Preparation and characterization of adsorbent
The starting material for the preparation of activated carbon was the dates stones of Phoenix dactylifera. L (Ghars variety) (Ouargla, Algeria), a waste agricultural product. Phosphoric acid was used for chemical activation. A ratio of (1:1.5) (w/w) powdered raw material and acid was prepared before distilled water was added in order to remove the impurities. Then, After 2 hours, the precursor was dried at 105˚C and heated to 450˚C. It was washed with distilled water, dried, and sieved to desired particle size 9.
The surface area and pore size of the adsorbent were measured by means of a nova surface area analyzer using a Brunauer–Emmett–Teller (BET) nitrogen adsorption technique. The average pore size and total pore volume were also determined in the same way. Table 1 displays the characteristics of adsorbent.
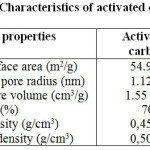 |
Table1: Character istics of activated carbon Click here to View table |
Adsorption experiments
The stock divalent cation solution (Pb++) was prepared by dissolving lead nitrate in bi-distilled water at 1000 mg/l. Working solutions were prepared by diluting different volumes of stock solution to achieve the desired concentration.
In the batch studies, 0.1g of the adsorbent was placed in a flask containing 20 ml of a lead solution with the desired concentration. The flask was continuously shaken (50 rpm) at a desired temperature and pH range (2–10). At the end of each step, the supernatant liquids were filtered and the Pb(II) concentrations were determined using an atomic adsorption spectrophotometer. The contact time was determined as the time required for the concentration of the lead in the solution to reach equilibrium (2h). The amount of metal adsorbed by the solid (q) and the percent of metal removal were calculated using the following equations
q = (C0 – Ce)V/m
metal ions removal (%) = (C0 – Ce)100 / C0
Where C0 is initial concentration of Pb(II) (mg/l), Ce is concentration of Pb(II) at equilibrium adsorption (mg/l), V is the volume of solution (l) and m is the mass of adsorbent (g).
The effect of temperature ranging from 25 to 60˚C was also studied. During the tests, the pH was adjusted to 6 and the contact time was set at 2h. The effect of pH (2 – 10) and the initial concentration of metal (5–50 mg/l) was also examined in order to determine its influence on the adsorption rate and also to obtain the adsorption isotherm. The shaking speed (50 rpm), the amount of adsorbent (0.1g), the contact time (2h), the pH = 6, and the temperature of 25˚C were kept constant.
Result and discussion
effect of pH
The removal of metal ions from aqueous solution by adsorption is highly dependent on the pH of the solution, which affects the surface charge on the adsorbent and the degree of ionization and speciation of the adsorbent. Most research reports that have been conducted on heavy metal sorption indicated that the decrease in ion sorption at acidic pH might be due to the increase in competition with protons on active sites. At alkaline pH, however, different effects may arise due to other processes such as the predominant presence of hydrated species of heavy metals, changes on the surface and precipitation of the appropriate salts5.
To check the effect of pH on Pb(II) adsorption using activated carbon prepared from dates stones as adsorbent, experiments were conducted by varying pH from 2 to 10 with an initial concentration of 50mg/l. The results obtained for adsorbent are shown in Figure 1.
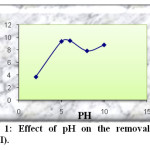 |
Fig1: Effect of pH on the removal of Pb(II). Click here to View Figure |
As can be seen initially, when the pH of solution was increased from 2 to 6, The amount of adsorbed Pb(II) increase from 3.72 to 9.45. The fact that the amount of Pb(II) removal at low pH is considerably lower may be accounted for by the competition between Pb++ and H+ ions the active sites on sorbent surface. The decreasing of the amount of adsorbed Pb(II) above pH = 6 due to formation of soluble hydroxyl complexes. It is assumed that OH– in the alkaline medium effects firstly hydrolysis products of Pb(OH)+, then affect Pb(OH)2 hydrolysis complexes, also, these effects decrease the adsorption. We can note that the optimal pH = 6.
Effect of contact time
The effect of the contact time on the adsorption Pb(II) by activated carbon is shown in figure 2. These data have been obtained from starting adsorbent and working solution without any pH adjustment (pH =6). The results showed that increasing the contact time increased the ions adsorption and then leveled up after 2h. Consequently, the contact time was set to 2h in each experiment. The amount of adsorbed lead by activated carbon from an initial concentration of 50 mg/l after 2 h was 9.91 mg/g.
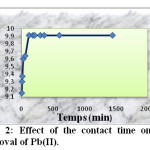 |
Fig2: Effect of the contact time on the removal of Pb(II). Click here to View Figure |
Effect of temperature
To investigate the effect of the temperature (25, 35, 45 and 60 °C) on the Pb(II) adsorption, the experiments were conducted with constant concentration of Pb(II) (50 mg/l), pH = 6, amount of activated carbon (0.1 g) and contact time (2 h). The results are given in figure 3.
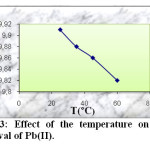 |
Fig3: Effect of the temperature on the removal of Pb(II). Click here to View Figure |
As can be seen from this figure, the adsorbed amount of Pb(II) ions decrease when increasing temperature from 25 to 60 °C.
The observed decrease in the adsorption capacity with an increase of temperature indicated that low temperature is in favour Pb(II) ions removal by adsorption onto activated carbon. This may be due to a tendency of the Pb(II) ions to escope from the solid phase to the bulk phase with an increase in the temperature of the solutions. This effect suggested that the adsorption mechanism associated with the removal of Pb(II) onto activated carbon involves a physical process, which is usually associated with low adsorption heat. This means that the adsorption process has an exothermic character.
Effect of initial concentration of metal
Dependency of the process of Pb(II) removal from different initial concentrations (5 – 50 mg/l) by the activated charbon is illustred in figure 4. The examination of the data reveals that the amount of absorbed lead increases with the concentration of solution from 0,98 mg/g to 9.91 mg/g. The percentage of adsorption increases also from 98.40 to 99.12 %.
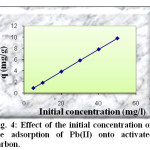 |
Fig4: Effect of the initial concentration on the adsorption of Pb(II) onto activated carbon. Click here to View Figure |
Adsorption isotherms
The equilibrium adsorption isotherms are of fundamental importance in the study and design of adsorption systems. According to the slop of the initial portion of the curves, they are classified into various groups. In this work, the isotherm curve corresponds to S-type in Gile’s classification (Figure 5).
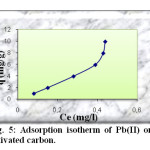 |
Fig5: Adsorption isotherm of Pb(II) onto activated carbon. Click here to View Figure |
Several methods have been published in the litterature to describe experimental data of adsorption isotherms. The Langmuir and Freundlich models are the most frequently employed models. In this work, both models were used to describe the relationship between the amount of Pb(II) ions adsorbed and its equilibrium concentration in solution at 25 °C , pH = 6 , for initial concentrations of 5, 10, 20, 30, 40 and 50 mg/l during 2h.
The linear form of the Langmuir isotherm model can be represented by the using equation below :
Ce/ qe = 1/qm b + (1/ qm) Ce
Where qe is the amount of solute adsorbed per unit weight of adsorbent (mg/g), Ce is the equilibrium of concentration of solute in the bulk solution (mg/l), qm is the monolayer adsorption capacity (mg/g) and b is the Langmuir constant related to the free energy of adsorption. The constants of Langmuir isotherm are obtained by plotting Ce/qe versus Ce (figure 6). They are given in the table 2.
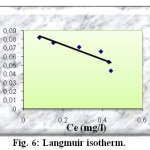 |
Fig6: Langmuir isotherm. Click here to View Figure |
The linear form of the Freundlich isotherm model is given by the following equation
Log qe = Log K + n Log Ce
Where K is the constant indicative of the relative adsorption capacity of the adsorbent (mg/g) and n is the constante indicative of the intensity of the adsorption. The constants of Freundlich isotherm are obtained by plotting Log qe versus Log Ce (figure 7). They are given also in the table 2.
 |
Table2: Constant of Freundlich and Langmuir in the adsorption of Pb(II) onto activated carbon Click here to View table |
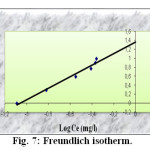 |
Fig7: Freundlich isotherm. Click here to View Figure |
The value of n (1.2831) represent a small but a beneficial adsorption of Pb(II) onto activated carbon.
The correlation coefficients indicate that the adsorption process conforms to the Freundlich model.
CONCLUSION
It can be concluded that activated carbons with high adsorption capacities can be produced from Algerian dates stones (Ghars variety) by phosphoric acid activation and can be employed as adsorbent for the removal of Lead (II) from aqueous solutions. However, the pH, time contact, temperature and initial concentration of metal could either affect the adsorption capacities of the activated carbon positively or negatively depending on the range of the parameters.
The experimental data of adsorption of Pb(II) followed the Freundlich equation.
REFERENCES
- Muther, I.K.; Jean-Luc, M. Journal of hazardous material, XXX, XXX – XXX.
- Rozada, F.; Otero, M.; Moran, A.; Garcia, A.I. Bioresource technol. 2008, 99, 6332 – 6338.
- Ahmed, J.; Lam,S.; Nora, A.A.; Noor,M.J.M.M. Desalination.2007, 206, 9 –16.
- Ucer, A.;Uyanik, A.; Aygun, S.F. Separ. Purify. Technol. 2006, 47, 113 – 118.
- Ibrahim,K.; Mehmet,U.; Hamdi, K.; Ali, C. Bioresource technol. 2008, 99, 429 – 501.
- Sreejale, K.G.; Anoop, K.; Anirudhan, T.S. Journal. Haza. mater. 2009 161,1506 – 1513.
- Ajay, K.M.; Mishra, G.K.; Rai, P. K.; Chita, R.; Nagar, P. N. Journal. Haza. mater B. 2009, 122,161 – 170.
- Zhang, K.; Cheung, W.H.; Valix, M. Chemosphere. 2005.60,1129 -1140.
- Chaouch, N, Ouahrani, M.R.; Chaouch C.; Gherraf, N. Desali. Water. Treat 2013, 51,2087–2092.
- Amuda, O.S.; A.A.Giwa, Bello, I.A. Biochem. Engine. J. 2007, 36,174 – 181.
- Anoop, A.k.; Anirudhan, T.T. Chem. Engine. J. 2008,137, 257 – 264
- Faur, C.B.; Kadirvelu, K. P. Carbon. 2002,40,2387 – 2392.
- Vivek, N.; Mahesh, G. Journal. Haza. mater. 2009,161, 575 – 580.
- Ayhan demirbas. Journal. Haza. mater. 2008,157, 220 – 229.
- Tonni, A.K. Chem. Engine. J. 2006, 118,83 – 98.
- Saffaj, N.; Loukili, H.; Alami,S. Y.; Albizane, A.; bouhria, M.; Persin, M.; Larbot, A. Desalination. 2004, 168, 301 – 306.
- Konoptiska, O.P. Applied. Surf. Sci. 2004,221,421 – 429.
- Kadirvelu, K, Faur, C. B. Langmuir. 2000, 16, 8404 -8409.
- Yosuk, K.; Qingrong, Q.; Motoi, M.; Hideki, T. Carbon. 2006, 44, 195 – 202.
- Mac Donald, J.A.F.; Quinn, D.F. Carbon. 2006, 34,1103 –1108.

This work is licensed under a Creative Commons Attribution 4.0 International License.









