The Irradiation of CO2 Ice at 30 K with Low Energy Ions
Sohan Jheeta
Open University, Department of Physical Chemistry, Walton Hall, Milton Keynes, MK7 6AA, UK.
DOI : http://dx.doi.org/10.13005/ojc/300201
Article Received on :
Article Accepted on :
Article Published : 11 Apr 2014
In space there are two types of chemistry, namely solid phase and gas phase; in this paper the latter chemistry formed the basis of our experimentations. An experimental investigation of the irradiation of CO2 ice at 30 K with low energy ions: H+ (1.5 keV), D+ (2.12 keV) and He+ (3 keV) was carried out under ultrahigh vacuum (10-9 mbar) conditions. Molecular products formed within the ice were detected and monitored using FTIR spectroscopy. The formation of CO was observed: this CO subsequently led to the synthesis of O3 and CO3, as well as tentative amounts of H2CO3 and D2CO3, the latter being the result of the process of implantation; no signals, tentative or otherwise, were observed during the irradiation with He+ ions. The consequences of these results for prebiotic chemistry in the interstellar medium and star forming regions are discussed.
KEYWORDS:Circumstellar disk, interstellar medium, interstellar dust particles, icy mantles, solid phase chemistry, ice modification, ions, low energy cosmic rays, low energy ions, secondary electrons
Download this article as:| Copy the following to cite this article: Jheeta S. The Irradiation of CO2 Ice at 30 K with Low Energy Ions. Orient J Chem 2014;30(2) |
| Copy the following to cite this URL: Jheeta S. The Irradiation of CO2 Ice at 30 K with Low Energy Ions. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=2853 |
Introduction
One of the intriguing scientific questions is: how molecules in the circumstellar disk and interstellar medium (ISM) are formed? Currently we know that there are over 195 (http://www.astrochymist.org/astrochymist_ism.html) molecules in these large scale structures in space, some with long chains (eg HC11N) and others containing a number of different atoms (eg isocyanic acid, HNCO). One answer to this question is that the icy mantles of interstellar dust particles (IDPs, it should be noted that the 80% of the IDPs are composed of silicates, followed by carbonaceous (20%) and remaining of minerals (eg CaAl12O19 in 0.002 ppm) in negligible amounts) are constantly irradiated with both electromagnetic and particle radiation, forming even more complex molecules such as amino acids – for example the amino acid glycine has been recently identified in the comet Wild 21. Reactions taking place within the icy mantles are said to occur in solid phase chemistry, which represents 25% of the chemistry occurring in the ISM, the remaining being assigned to gas phase chemistry.
Universe-wide, the ions H+, D+ and He+ (including their atomic counterparts and e–) are dominant in terms of particle radiation (99%), with energies in the region of 1-10 MeV and are termed as low energy cosmic rays2,3. Such ions can penetrate deep into the interior of huge dark molecular clouds are dark because primarily they are made of molecular hydrogen and IDPs with a ratio of 1012 hydrogen atoms to 1 IDP6, as in Figure 1 (Horsehead nebula) and, in the process, their energy drops to below 5 keV as they hurtle deeper into the interior of the ISM; these low energy particles impinge onto the icy mantles of IDPs4.
 |
Figure 1: shows an example of a dark molecular cloud, the Horsehead nebula. (Credit: http://www.nasa.gov/multimedia/imagegallery/image_feature_89.html) |
The reason IDPs have icy mantles is because the interior of the clouds have temperatures in the region of 10 to 50 K5,6 and, at such temperatures, volatiles (eg H2O, CO, CO2, NH3, CH4 etc) freeze out on the surface of the IDPs forming volatiles-rich icy mantles4. Low energy particles are involved in the processing of ices of comets; asteroids; objects belonging to the Kuiper Belt and Oort cloud; atmospheres and surfaces of planetary bodies – planets themselves as well as their satellites. For example, the aerosol content of Titan’s atmosphere is constantly being irradiated by ions from the magnetosphere of Saturn7,8 and in the process Titan’s atmosphere (and its surface) contains a rich source of organic compounds9.
Solid carbon dioxide (CO2) was first detected in the ISM in 198910. Since then it has been affirmed that CO2 is the second most common molecule within the ISM as well as in comets11 (Table 1).
| Species | Protostars | Comets |
| Water | 100% | 100% |
| CO | ~15 | 7-20 |
| CO2 | ~23 | 3-6 |
| NH3 | ~8 | 1.5 |
| O2 | <7 | <0.5 |
| CH3OH | ~6 | ~2 |
| HCOOH | ~3 | ~0.05 |
| H2CO | <3 | ~3 |
| CH4 | ~2 | ~1 |
| C2H6 | <0.4 | ~0.5 |
| OCS | <0.04 | 0.5 |
|
Taken from TL Roush (2001) |
||
Table 1: show the distribution of some common molecules in protostars and comets
It is formed on the mantle of IDPs during the photolysis and radiolysis of pure CO, CO:O2, and CO:H2O ices. Possible formation reactions include: pure CO reaction: via the dissociation of CO4.

CO:O2 reaction: via the dissociation of O2.

CO:H2O reaction: via the dissociation of H2O2 (the most prevalent reactions occur in this mixture).

Such reactions generally occur in structures like the Horsehead nebula.
From a solid phase chemistry point of view, many other compounds can also be made from CO2 via CO (Eq. 7) within the ISM. For example, the following sequence of reactions during the formation of formaldehyde (H2CO) – biologically a very important compound in the synthesis of sugars – is possible12.
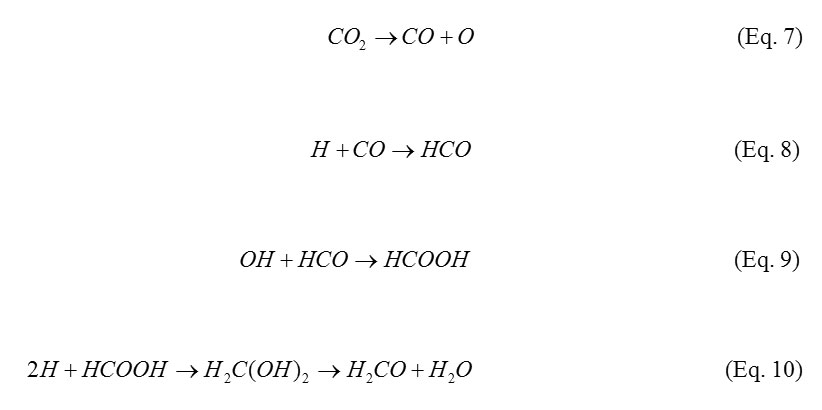
This is one reason why CO2 was chosen to study its astrochemistry. The aim of this experiment is to investigate the effect of the low energy ions, H+, D+ and He+ on CO2 ice at 30 K, whilst keeping their charge at +1.
Method
These experiments were carried out at Queens University, Belfast (QUB) which is the site of the UK’s low energy ion beam facilities. The Electron Cyclotron Resonance Ion Source (ECRIS) at QUB is used to produce multiple charged ions using a 9.0-10.5 GHz ECRIS13. Beams of H+, D+ or He+ ions are extracted from the 9.0-10.5 GHz ion source. A low energy ‘floating’ beamline accelerator is then used to extract the ions by holding the beamline at 4 keV potential. The generated ions are controlled by powerful magnets with the net effect that ions are separated according to mass:charge ratio (m/Q). The ions are then focussed into the vacuum chamber by a series of deflection/focussing plates. Before irradiating the ice, the ion current was measured using a Faraday cap placed in the path of the ion beam – that is, between the origin of the beam and the vacuum chamber. During irradiation, any changes in the beam current were continuously monitored using a 90% transmitting mesh placed in the beam path in place of the Faraday cap (Figure 2).
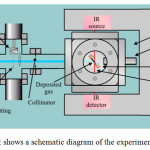 |
Figure 2: shows a schematic diagram of the experimental setup |
A general description of the vacuum chamber is given in Dawes14 (2007). Ices of CO2 (99.995%, Argo International) were prepared at 30 K before irradiating them with appropriate radiation ions of 1.5 keV H+ (300 nAmp), 2.12 keV D+ (4 mAmp) or 3 keV He+ (400 nAmp). Analysis of the irradiated CO2 ice was carried out using in situ infrared spectroscopy derived from a N2 purged Thermo-Nicolet Nexus 670 Fourier Transform Infrared (FTIR) spectrometer. Spectra were acquired in the range from 4 000 to 650 cm-1 at 4 cm-1 resolution. The sample was positioned with the infrared and ion beams perpendicular to one another and both at 45° to the substrate surface (Figure 2).
The thickness of the ices was calculated using the following equations:
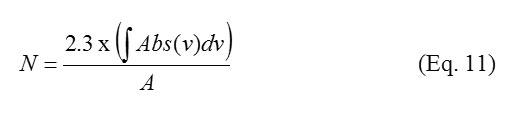
Where ∫Abs(v)dv= measure of band strength in cm-1 and A = integral absorption value cm molecules; and

Additional information required to calculate the thickness (d) of CO2 ice is as follows: density (ρ) of CO2 ice: 1.7 gcm-3(15); and CO2 integrated absorption coefficient (A value) at 3708 cm-1: 2.5 x 10-18 cm molecule-1(16). The thickness of the ice obtained for 1.5 keV, 2.12 keV and 3.0 keV irradiation experiments were: 1.7mm, 2.1mm, and 2.1mm respectively.
Results
Table 2 shows the new species observed when CO2 ice was irradiated with different types of radiation (H+, D+ or He+) with different energy levels. The obtained results are compared with those generated by irradiation using UV light17 (photolysis) and by radiolysis18.
|
Molecules and radicals observed |
This work: Radiolysis of CO2 ice |
Gerakines (1996) Photolysis of CO2 ice. UV Light |
Brucato (1997) Radiolysis of CO2 ice. 1.5 keV H+ |
||
|
Radiolysis: 1.5 keV H+ |
Radiolysis: 2.12 keV D+ |
Radiolysis:3 keV He+ | |||
|
v = cm-1 |
v = cm-1 |
v = cm-1 |
v = cm-1 |
v = cm-1 |
|
| O3 |
706 |
||||
| CO3 |
973 |
976 |
|||
| O3 |
1043 |
1043 |
1043 |
1043 |
1040 (H2CO3;O3) |
| Unidentified |
1053 |
||||
| CO3 |
1078 |
1078 |
1078 |
1067 |
|
| H2CO3 |
1302 |
||||
| H2CO3 |
1490 |
||||
| H2CO3 |
1736 |
||||
| CO3 |
1880 |
1880 |
1880 |
1883 |
1879 (HCO?) |
| CO3 |
2044 |
2044 |
2044 |
2044 |
2044 |
| 13CO |
2093 (weak) |
2093 |
2093 |
2093 |
2094 (weak) |
| CO |
2141 |
2141 |
2141 |
2141 |
2143 |
Table 2 shows identification of bands produced during irradiation with different ions
Figure 3 depicts a section of recorded spectra when the CO2 ice at 30 K was irradiated with H+ ions, to illustrate the formation of new species of molecules.
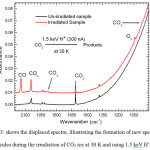 |
Figure 3: shows the displaced spectra, illustrating the formation of new species of molecules during the irradiation of CO2 ice at 30 K and using 1.5 keV H+ ions |
The three major species of interest observed during the irradiation of CO2 ice at 30 K with H+, D+ or He+ ions were: carbon monoxide (CO) at v = 2141 cm-1, ozone (O3) at v = 1043 cm-1 and carbonate (CO3) at v = 1078 cm-1. In all three cases the greatest and least amount of products generated were CO and CO3 respectively, with O3 being in the middle range (Figures 4a, b and c).
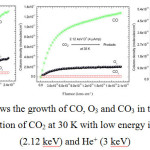 |
Figures 4a, b and c: shows the growth of CO, O3 and CO3 in terms of column density (N) during the irradiation of CO2 at 30 K with low energy ions: H+ (1.5 keV), D+ (2.12 keV) and He+ (3 keV) |
CO synthesis, a primary product, is essentially as in Eq. 7. The secondary products, O3 and CO3, are made from reaction with O produced during the radiolysis of CO2 as follows:

and CO3 as below:
![]()
The information from Figures 4a, b and c has been re-presented to show the growth of each new species of molecule with respect to the radiations deployed (Figures 5a, b and c) – for example, Figure 5a shows the growth of CO with respect to all three radiations.
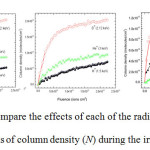 |
Figures 5a, b and c: compare the effects of each of the radiations on the growth of CO, O3 and CO3 in terms of column density (N) during the irradiation of CO2 at 30 K |
By studying the column densities in Table 3 and Figures 5a, b and c, it can be noted that D+ is efficient at making CO, O3 and CO3; Figures 5a and b shows that He+ is good at producing reasonable amounts of CO and O3 more than H+, but less than D+; lastly Figure 5c, depicts that H+ is only effective at making CO3.
|
Column density (N) molecules cm-2 |
|||
|
CO |
O3 |
CO3* |
|
|
1.5 keV H+ (Fluence: 1.87×1012) |
3.0×1016 |
8.0×1015 |
1.0×1015 |
|
2.12 keV D+ (Fluence: 2.50×1013) |
1.3×1017 |
2.0×1016 |
8.8×1015 |
|
3.0 keV He+ (Fluence: 2.50×1012) |
5.0×1016 |
9.0×1015 |
1.6×1014 |
|
*estimated from Figure 6. The smooth curves in Figure 6 were obtained by using Origin software (version 6). |
|||
Table 3: shows final column densities levels (molecules cm-2) during the irradiation of CO2 ice at 30 K with H+, D+ and He+ radiations.
Discussion
The incoming ions collide with the CO2 ice, causing the bonds to break. The type and quantity of CO2 bond-breaking depends on the nature of the radiation (electromagnetic or particle); the fluence – the number of particles impinging onto the given surface area; the molecular composition of the ice, which will determine total stopping power of the ice; and the nature of the incoming particles, including whether these are neutral (), mono (He+, ) or multivalent (He2+, C3+ etc), what their energy levels are, and their mass-to-charge ratio – all of which determines their depth of penetration and bond breaking efficiency. For the duration of ionic irradiation, as soon as the ion impinges the ice, it begins to lose energy and will come to a halt either on the surface (causing sputtering) or within the ice itself. If it is the latter, the following modifications may result: electronic transitions of the ice molecules; ionisation of the ice molecules, leading to a generation of high energy secondary electrons that go on to ionise and excite other molecules, in the process modify the ice further; or the collision may also dissociate the ice molecules, which will add to the inventory of products that can be made.
During the low energy irradiation of CO2 ice at 30 K with H+, D+ or He+ ions, fluence was responsible for the destruction of CO2. D+ ions are more efficient at breaking CO2 bonds compared to the H+ or He+ ions as shown by the column densities of destruction products (CO, CO3 and CO3) in Table 3 and also as testified by Figures 5a, b and c. This is because D+ ions have the highest fluence (2.50 x 1013 D+ cm-2), even though they have lower energy than He+.
It is highly probable that the He+ ions are sputtering on the surface of the CO2 ice, perhaps this may be one reason for the results as depicted in Figure 5c, ie it has the lowest column density for CO3. As the helium ion’s cross section (s) is four times the size of that of the hydrogen ion (m/Q ratio for He+ = 4:1; H+ = 1:1), suggesting that He+ may not be penetrating the ice upon impinging – ie causing sputtering on the surface of the ice, thereby releasing the trapped CO and O3 as observed in Figure 5a and b.
Figure 5c also compares CO3 generated during the irradiation of CO2 with the three types of ionic radiation. It is probable that the formation of CO3, in addition to being a less efficient reaction, is being removed from the system altogether.
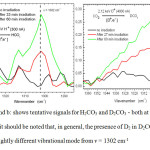 |
Figures 6a and b: shows tentative signals for H2CO3 and D2CO3 – both at v = 1302 cm-1. However, it should be noted that, in general, the presence of D2 in D2CO3 should produce a slightly different vibrational mode from v = 1302 cm-1 Click here to View figure |
Figure 6a (above) shows the possible, tentative signals for H2CO3, a product also reported by Brucato and co-workers18 (1997) as in Table 2, meaning that CO3, during H+ irradiation, is being steadily removed via the Eq. 16, thus producing a curve as shown in Figure 5c.
![]()
During D+ irradiation (Figure 5c), the curve at 2.5×1014 molecules cm-2 fluence levels off, again suggesting that D2CO3 (tentative signals indicated in Figure 6b) is being formed (Eq.17) then rapidly removed.
![]()
This may be speculative because, in general, H+ and D+ would have slightly different vibrational wavenumbers – ie both would not necessarily have v = 1302 cm-1.
Both reactions in Eq. 16 and Eq. 17 are examples of implantation, a process whereby incident radiation partakes in the chemical reactions18.
Astrobiological implications
In this and other experiments17,18, the same new molecules were formed regardless of the type of irradiation used (Table 2). Such species of molecules are also part and parcel of the ISM, present in the planetary atmospheres and surfaces, as well as in comets and asteroids. CO2 and its products are important as far as astrochemistry is concerned, for example as stated in the introduction, formaldehyde and formic acid can both be produced from CO2. Both of these compounds are important during the formation of other biologically important molecules such as those belonging to the groups: carbohydrates, amino acids, nucleotides and cofactors (ATP, acetyl-CoA, NADH, etc). As a result, the reality is that CO2 (and C chemistry in general) is very important eg during photosynthesis and we have to explore all avenues that may help to answer the more difficult question: how did life on Earth actually get started? CO2, and its destruction products and downstream compounds could have been delivered onto the Earth during the bombardment phase of its early history (~4.3-4.0×109 years ago) and may thus have been instrumental in the inception of the origins of life on Earth.
Conclusion
The destruction of CO2 led to the formation of CO and O, which in turn led to the formation of the secondary molecules O3 and CO3. These species were observed during the irradiation of CO2 ice at 30 K with 1.5 keV H+, 2.12 keV D+ and 3 keV He+ ions. In addition, the following summary conclusion was also reached: fluence resulted in the modification of ice, which produced new species; the cross section of the ion as well as the stopping power of the ice determines the degree to which the impinging particle can penetrate the ice; and particles with smaller cross sections (eg H+) could partake in the ice modification as they penetrate deeper within the ice – eg formation of H2CO3 and D2CO3 by the impinging of H+ and D+ ions respectively – a process known as implantation.
In relation to the discovery of life’s astrobiological beginnings, the study of solid phase chemistry is important in recreating the formation of molecules under space conditions, thus demonstrating the possibility that these molecules would have been present during Earth’s early history and thus were available to play a part in the beginnings of the origin of life.
Acknowledgements
We would like to thank both the STFC and EU lassie project as well as Professor Robert W McCullough for making the facilities available at his laboratory for conducting the entitled above experiment. We also extend thanks to the Queen’s University, Belfast, BT7 1NN.
References
- Elsila E., Glavin, D. P., Dworkin, J. P., Meteoritics & Planet. Sci. 44(9), 1323-1330 (2009).
- Hudson, R. L., Moore, M. H., J. Geophys.l Res. Planets106(E12). 33275-33284 (2001).
- Tielens, A., “The Physics and Chemistry of the Interstellar Medium.” Cambridge University Press, England”, 168, (2005).
- Gerakines, P. A., Moore M. H., Hudson R. L., J Geophys. Res. 106 (E12), 33381-33386, (2001).
- Strazzulla, G., Baratta G. A., Palumbo M. E., Spectrochimica Acta Pt. a-Molecu. & Biomolec. Spectroscopy 57(4): 825-842 (2001).
- Snow, T. P., McCall, B. J., Ann. Rev. of Astron. & Astrophys. 44: 367-414, (2006).
- Johnson, R. E. “Energetic charged-particle interaction with atmospheres and surfaces.” Publishers: Springer-Verlag, New York, 232 (1990).
- Delitsky, M. L., Lane, A. L., J. Geophys. Res. Planets 107(E11), (2002).
- Raulin, F., Owen, T., Space Science Rev. 104(1-2), 377-394. (2002).
- Dhendecourt, L. B., and De muizon, M. J., A & A 223(1-2): L5-L8, (1989).
- Roush, T. L., J Geophys. Res. Planets, 106(E12), 33315-33323, (2001).
- Henkel, C., Mauersberger R., Wiklind, T., Huttemeister, S., Lemme, C., Millar, T. J., A & A., 268(2): L17-L20. (1993).
- Broetz, F., Trassl, R. W., McCullough, R., Arnold, W., Salzborn, E., Physica Scripta, T92, 278-280, (2001).
- Dawes, A., Mukerji, R. J., Davis, M. P., Holtom, P. D., Webb, S. M, Sivaraman, B., J Chem. Phys., 126(24), (2007).
- Holtom, P. D. PhD Thesis – University College London, 201, (2005).
- Hudgins, D. M., Sandford, S. A., Allamandola, L. J., Tielens, A. G. G. M., The APJ Suppliments Series, 86,713-870, (1993).
- Gerakines, P. A., Schutte W. A., Ehrenfreund, P., A & A 312(1), 289-305, (1996).
- Brucato, J. R., Castorina, A. C., Palumbo, M. E., Satorre, M. A., Strazulla, G., Planet. & Sp. Sci. 45(7), 835-840, (1997).

This work is licensed under a Creative Commons Attribution 4.0 International License.









