Removal of azo dye from aqueous solutions using chitosan
1Zuhair Jabbar, A , Angham*2, G. Hadi Ferdoos Sami2
1Babylon University, College of Engine Materials. Iraq
2,3Babylon University, College of Science, Chemistry Department. Iraq
DOI : http://dx.doi.org/10.13005/ojc/300222
Article Received on :
Article Accepted on :
Article Published : 06 Jun 2014
Adsorption of Congo Red (CR) from aqueous solution onto chitosan was investigated in a batch system. The effects of solution pH, initial dye concentration, and temperature were studied. Results indicated that chitosan could be used as a biosorbent to remove the azo dyes from contaminated water. Synthesize of chitosan involved three main stages as preconditioning, demineralization, deproteinization and deacetylation. Chitosan was characterized using Fourier Transform Infrared Spectroscopy (FTIR) and solubility in 1% acetic acid.
KEYWORDS:Adsorption; Congo red; Chitosan; synthesized.
Download this article as:| Copy the following to cite this article: Jabbar Z, Angham A, Sami G. H. F. Removal of azo dye from aqueous solutions using chitosan. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Jabbar Z, Angham A, Sami G. H. F. Removal of azo dye from aqueous solutions using chitosan. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3680 |
Introduction
The chemical contamination of water from a wide range of toxic derivatives is an important environmental problem [1]. Synthetic dyes usually have a complex aromatic molecular structure, which possibly comes from coal-tar based hydrocarbons such as benzene, naphthalene, toluene, xylene, etc. The complex aromatic molecular structures of dyes make them more stable and more difficult to biodegrade. [2–4]. It is known that wastewaters containing dyes are very difficult to treat, since the dyes are recalcitrant molecules (particularly azo dyes), resistant to aerobic digestion, and are stable to oxidizing agents. Textile, paper, plastics, and cosmetic industries use a wide variety of dyes to color their products and discharge large amount of effluents including dyes which are very toxic and could cause serious ecological problems. Therefore, dye pollution in water stream is a major environmental problem.[5].
The methods of dye removal from industrial wastewaters could require many processes such as biological treatment, coagulation, electrochemical techniques, adsorption, and oxidation. Among these methods, adsorption is considered an effective and economical method to remove dyes from wastewaters.[6-11]. It has been reported that many different types of adsorbents are effective in removing color from aqueous effluents. Natural polymeric materials are gaining more and more interest for application as adsorbents in wastewater treatment due to their biodegradable and non-toxic nature. Currently, the most common procedure involves the use of activated carbon [12]. Activated carbon is regarded as an effective but expensive adsorbent due to the high cost of manufacturing and regeneration. Because of its relatively high cost, there have been attempts to utilize low cost and naturally occurring adsorbents. There are many different studies on the use of low cost materials such as various agricultural wastes [13–15], Chitosan (CS) offers an interesting set of characteristics, including non-toxicity, biodegradability, biocompatibility, and bioactivity.
Chitosan and its derivatives have been extensively investigated as biosorbents ( figure 1) for removal of heavy metals and dyes.[16-20]
![Figure 1. Chemical structures of chitin, chitosan and cellulose [21] .](http://www.orientjchem.org/wp-content/uploads/2014/06/Vol30_No2_Removal_Zuh_fig1-150x150.jpg) |
Figure1: Chemical structures of chitin, chitosan and cellulose [21] . |
The overall objective of this study is to investigate the dye removal from wastewater by adsorption using chitosan. The specific objectives included; synthesize chitosan from fish shells and removal of Congo red dye using as a bio-adsorption material.
Materials and methods
Preparation of Sorbent
Traditional isolation of chitin consists of four traditional steps (Figure 2): demineralization (DM), deproteinization (DP), decolorization (DC), and deacetylation (DA).
![Figure 2. Traditional Chitosan Production [22].](http://www.orientjchem.org/wp-content/uploads/2014/06/Vol30_No2_Removal_Zuh_fig2-150x150.jpg) |
Figure 2: Traditional Chitosan Production [22].Click here to View Figure |
Preparation of Adsorbent
Congo red contains an azo (-N=N-) chromophore and an acidic auxochrome (sulfonate : -SO3H) (which, respectively, gives and reinforces the coloration) associated with the benzene structure. Congo red is a the sodium salt of a derivative of benzidine and naphthionic acid as in figure 3.
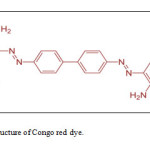 |
Figure (3): Chemical structure of Congo red dye. Click here to View Figure |
The physiochemical properties of this dye can be shown in table(1)
|
Parameter |
Value |
|
Molecular formula |
C32H22N6Na2O6S2 |
|
Molecular weight |
696.665 |
|
Maximum adsorption |
498 nm |
|
Nature |
Anionic dye |
Batch adsorption experiments
A stock solution of CR (1.0 g/L) was prepared by dissolving 1 g of CR powder in 1 L of double distilled water. The desired concentrations ranging from 10 to 60 mg/L were obtained by dilution. For each adsorption experiment, 50 ml of the dye solution with a specified concentration was stirred at 100 rpm in a glass flask. The pH of solutions was adjusted to a desired value by adding 0.1 mol/L NaOH or HCl solution. Batch adsorption experiments were carried out using a thermostated shaker for a certain contact time at a determined temperature at 100 rpm. At predetermined time intervals, samples were withdrawn by a pipette and centrifuged at 4000 rpm. Then, the residual concentration was determined from a constructed calibration curve by measuring the absorbance at λmax = 498 nm using UV-Vis spectrophotometer.
Batch adsorption experiments were carried out to examine effects of adsorbent dosage, initial dye concentration, solution pH, and time on the adsorption of CR on chitosan.
The amount of CR adsorbed on chitosan (at a predetermined time t), qt (in mg/g), was determined using the mass balance equation
qt = (Co – C t) x m/v…………………..(1)
The decolorization rate (η) of CR was calculated by the following equation
μ = (C0 Ct)/ C0 x 1000%………………(2)
where C0 is the initial concentration of CR (in mg/L), Ct (in mg/L) is the instant concentration of CR at a predetermined time t, V is the volume of the solution (in L), and m is the mass chitosan (in g).
Results and discussion
Effect of adsorbent dosage
The effect of adsorbent dosage (varied from 0.025 to 0.25 gm) on the percentage removal of 50 mg/L CR solution is shown in Fig. 3. The percentage removal of CR from the solution increased from 30% to 86% as the adsorbent dosage increased from 0.025 to 0.25 gm. This result is expected because of the increased adsorbent surface area and availability of more adsorption sites caused by increasing adsorbent dosage[23,24]
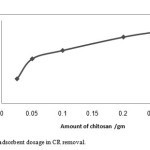 |
Figure 3(B): Effect of adsorbent dosage in CR removal. Click here to View Figure |
Effect of solution pH
The pH of the dye solution affects the surface charge of the adsorbent, the degree of ionization of the materials, and the dissociation of functional groups on the active sites of the adsorbent. As well, it affects the structure of the dye molecule[25]. The percentage removal of CR at different pH values is plotted in Fig. 4. The percentage removal increased from 24.29% to 80.85% when pH was decreased from 10 to 2. This was because the CR molecule (with one sulfuric group) ionized easily even in acidic media and became a soluble CR anion. As the initial solution pH decreased, the number of positively charged active sites increased due to the protonation of the amine groups (–NH2) in the CS chain. Consequently, the electrostatic interaction between the positively charged adsorbent and the CR anions increased, which resulted in increased adsorption. Furthermore, the lower adsorption removal percentage at alkaline pH contributed to the presence of excess hydroxyl ions, which competed with the CR molecules for adsorption sites [23]. In contrast, as expressed in equilibrium equation (3), the quinoid structure of CR is more easily reduced than its azo structure [26]. Therefore, higher removal percentages were observed at acidic pH values for CR adsorption.
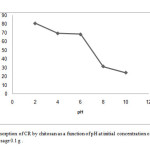 |
Figure 4: Adsorption of CR by chitosan as a function of pH at initial concentration of 50mg/L and adsorbent dosage 0.1 g . Click here to View Figure |
Effect of contact time
A 50ml of 50mg/L of CR dye was taken in conical flasks and treated with 0.1 gm chitosan (adsorbent) at several times (20, 40, 60, 80, 100, 120 and 140 min.). the variation in percent removal of dye with the time was shown in figure 5. The percentage removal increased from 31.41% to 80.60% when time was increased from 20 to 140 min., this due to saturation of active sites which do not allow further adsorption to take place[27].
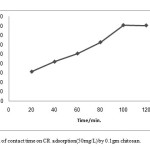 |
Figure 5: Effect of contact time on CR adsorption(50mg/L) by 0.1gm chitosan. |
Effect of initial CR concentration
The effect of initial CR concentration on the percentage removal of the dye is shown in figure 6. The initial CR concentration was varied from 10 to 60 mg/L. A rapid initial adsorption of CR took place within the first 20 min, after which the adsorption slowed down and then almost reached equilibrium at 120 min. The percentage of CR removal evidently decreased with increasing initial dye concentration. The percentage removal was 82.05% for 10 mg/L initial concentration, and only 30.26% for 60 mg/L after 120 min of adsorption (figure 6). This was caused by an increase in the mass gradient pressure between the solution and adsorbent. The gradient acted as the force that drove the transfer of the dye molecules from the bulk solution to the particle surface[23] .
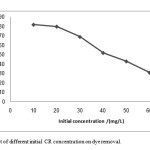 |
Figure 6: Effect of different initial CR concentration on dye removal. Click here to View Figure |
Conclusions
The synthesize of chitosan involved three main stages demineralization, deproteinization, and deacetylation., it characterized by using Fourier Transform Infrared Spectroscopy (FTIR) and solubility in 1% acetic acid. CR adsorption onto the chitosan depended highly on adsorbent dosage, initial dye concentration, solution pH and time.
References
- V. Singh, A. K. Sharma, D. N. Tripathi, R. Sanghi, Poly(methylmethacrylate) Grafted Chitosan: An Efficient Adsorbent, J. Hazard. Mater. 2009, 161, 955–966.
- C. A. Fewson, Biodegradation of Xenobiotic and Other Persistent Compounds: The Causes of Recalcitrance, Trends Biotechnol. 1988, 6, 148–153.
- S. Seshadri, P. L. Bishop, A. M. Agha, Anaerobic/Aerobic Treatment of Selected Azo Dyes in Wastewater, Waste Manage. 1994, 15, 127–137.
- P. Nigam, G. Armour, I. M. Banat, D. Singh, R. Marchant, Physical Removal of Textile Dyes from Effluents and Solid-State Fermentation of Dye-Adsorbed Agricultural Residues, Bioresour. Technol. 2000, 72, 219–226.
- M. M. Perju and E. S. Dragan, Removal of azo dyes from aqueous solutions using chitosan based composite hydrogels, iel, 2010, 3,7-11.
- Chiou M. S., Li H. Y., Chemosphere 50 (2003) 1095-1105.
- Rauf M. A., Bukallah S. B., Hamour F. A., Nasir A. S., Chem. Eng. J. 137 (2008) 238- 243.
- Mittal A., Mittal J., Malviya A., Kaur D., Gupta V. K., J. Colloid Interface Sci. 343 , (2010) 463-473.
- Chakraborty S., De S., DasGupta S., Basu J. K., Chemosphere 58 (2005) 1079-1086.
- Dragan S., Cristea M., Arinei A., Poinescu Ig., Luca C., J. Appl. Polym. Sci. 55 (1995) 421-430.
- Wawrzkiewicz M., H, Hubicki Z., Chem. Eng. J. 157 (2010) 29-34.
- T. Calvete, E. C. Lima, N. F. Cardoso, S. L. P. Dias, E. S. Ribeiro, Removal of Brilliant Green Dye from Aqueous Solutions Using Home Made Activated Carbons, Clean – Soil Air Water 2010, 38 (5–6), 521–532.
- P. Saha, S. Chowdhury, S. Gupta, I. Kumar, R. Kumar, Assessment on the Removal of Malachite Green Using Tamarind Fruit Shell as Biosorbent, Clean – Soil Air Water 2010, 38 (5–6), 437–445.
- Z. Aksu, S. Tezer, Equilibrium and Kinetic Modeling of Biosorption of Remazol Black B by Rhizopus arrhizus in a Batch System: Effect of Temperature, Process Biochem. 2000, 36 (5), 431–439.
- Z. Aksu, Biosorption of Reactive Dyes by Dried Activated Sludge: Equilibrium and Kinetic Modeling, Biochem. Eng. J. 2001, 7 (1), 79–84.
- Crini G., Badot P.-M., Prog.Polym.Sci. 33 (2008), 399-447.
- Dragan E. S., Dinu M. V., Timpu D., Bioresour. Technol .101 (2010) 812-817.
- Chatterjee S., Chatterjee S., Chatterjee B. P., Guha A. K., Colloids Surf. A 299 (2007) 146-152.
- Angham G. Hadi,(2012), Adsorption of Cd(II) ions by synthesize chitosan from fish shells, British journal of science,Vol.5(2).
- Angham G. Hadi,(2012), Study of heavy metal Mn2+ adsorption by synthesized chitosan, BJS,6(2)
- WEB_4,2007.Dalwoo-ChitosanCorporation, http;// members.tripod.com/Dalwoo/structure.
- Meyers, S.P. and No, H.K. 1995. Utilization of crawfish pigment and other fishery processing by-products. Ch. 20. In “Nutrition and Utilization Technology in Aquaculture,” Lim, C.E and Sessa, D.J. (Eds.), p. 269-277. AOCS Press, Champaign, IL.
- L. Wang, J. Zhang, R. Zhao, C. Li, Y. Li, C.L. Zhang, Adsorption of basic dyes on activated carbon prepared from Polygonum orientale Linn: equilibrium, kinetic and thermodynamic studies, Desalination, 254 (1–3) (2010), pp. 68–74.
- P.S. Kumar, S. Ramalingam, C. Senthamarai, M. Niranjanaa, P. Vijayalakshmi, S. Sivanesan, Adsorption of dye from aqueous solution by cashew nut shell: studies on equilibrium isotherm, kinetics and thermodynamics of interactions, Desalination, 261 (1–2) (2010), pp. 52–60.
- G. Crini, H.N. Peindy, F. Gimbert, C. Robert, Removal of C.I. Basic Green 4 (malachite green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies, Sep. Purif. Technol., 53 (1) (2007), pp. 97–110.
- V.F. Chemie, New methods for determination of β-lactam antibiotics by means of diffuse reflectance spectroscopy using polyurethane foam as sorbent, PhD thesis. Duisburg University, Germany, 2005.
- W. Nakbanpote,P. Thiravavetyan and C. Kalambaheti, Mineral Eng.,13,391-552(2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.









