Preliminary Study of Zinc Removal from Cyanide-free Alkaline Electroplating Effluent by Precipitation Using Oxalis Plants
Benouali D.*1, Kherici S.2, Belabbassi M.1, Belkandouci M.M.1, Bennemra A.1
1Department of Physical Chemistry, Faculty of Chemistry, USTO- ALGERIA
2Department of Industrial organic Chemistry,Faculty of Chemistry, USTO- ALGERIA
DOI : http://dx.doi.org/10.13005/ojc/300215
Article Received on :
Article Accepted on :
Article Published : 04 Jun 2014
The aim of this study is the reduction of Zinc from the cyanide-free alkaline zinc-platingeffluent. Various methods utilizedfor removing or recovering the heavy metals are adsorption, chemical precipitation, sedimentationfiltration, reverse osmosis etc. We are interested in using oxalisplant as a potential chelating/settling agent for the treatment of electroplatingeffluent collected from a local industrial unit.Oxalis pes-capaehas an exceptionally high content of oxalic acid which isknown as moderate and week chelating agent.Theefficiency of the performance oxalis powder (KITININ)was estimatedand the optimum dosewasdeterminedusing conductivity, volumetric, atomic absorption spectroscopy and pH measurements. The results show the performance of oxalis in treating Zn plating effluent effectively.In effect, a white crystalline precipitate of zinc oxalate is easily extracted(98,52 % elimination of Zn) and ahighsettling rateis observed.The maximum zinc uptake by Oxalis in batch systems is estimated to6,44 mg/g.
KEYWORDS:Oxalis; precipitation; Zinc; electroplating effluent
Download this article as:| Copy the following to cite this article: Benouali D, Kherici S, Belabbassi M, Belkandouci M. M, Bennemra A. Preliminary Study of ZincRemoval from Cyanide-free Alkaline Electroplating Effluent by Precipitation Using Oxalis Plants. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Benouali D, Kherici S, Belabbassi M, Belkandouci M. M, Bennemra A. Preliminary Study of ZincRemoval from Cyanide-free Alkaline Electroplating Effluent by Precipitation Using Oxalis Plants. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3548 |
Introduction
Today,developed industries can use more than 40% of total water, leading to more water quality problems. The metal pollutants can originate from a variety of sources: Metal finishing and electroplating processes. The plating baths contain high concentrations of potential polluting metals. The rinse water has a low concentration of heavy metals but, can contribute to significant load because of their large volumes. In addition only 30-40% of all metals used in the electroplating process can be effectively plated onto the articles[1]. Its residual waste effluents are responsible for the supply of toxic and corrosive heavy metals to the environment. So, a number of treatment techniques have been developed and are utilized for heavy metal removal or recovery [2], because of the slow natural process of metal formation(metals are considered to be a nonrenewable resource) and their toxic effects [3, 4]. These points have clearly explained the importance of fixing some limits for these heavy metals in drinking and waste waters.Treatment methods to reach the fixed limit (5ppm) include chemical precipitation and other separation methods [5-12]. In this study we are interested in the treatment of the cyanide-free alkaline zinc-plating effluent, where cyanide may be replaced by complexing or chelating agents such as sodium gluconate, triethanolamine or polymeric amines. The resulting baths present therfore problems with waste treatment. A second generation of organic addition agents eliminated chelating agents was developed in industry and most of the currently available processes have eliminated these problems with the use of an entirely new family of organic reaction products [13] but zinc removal from dilute solution remains a problem. Many researchers have studied various natural and synthetic chelating agents for their ability to remove heavy metals from electroplating effluents. Chelating agents(organic acids) are the most popular extracting reagents used forsoils decontamination [14,15]. Oxalate compound used to precipitate metal ions fromsolutions,oxalate-treated activated carbons was also used for the adsorption of Co and Ni from aqueous solutions [16]. The aim of the present study is to assess the potential of a natural, low molecular weight organic acid,oxalic acid for zinc removal from electroplating effluent.This aliphatic organic acide xists naturally form relatively stable complexes with metals and has a greater potential to mobilize Zn from aqueous effluent in its insoluble zinc oxalate form. Indigenous to South Africa, Oxalis pes-caprae isan invasive plant in many other parts of the world, including Algeria and it can contain up to 16% of the dry weight as oxalic acid, present in both photosynthetic and non-photosynthetic tissues[17]. The present study provides general guide lines for the removal of dissolved Zn metal from cyanide-free electroplating effluent using oxalis plant in a powder form (KITININ) estimated as a potential chelating/settling agent.
Material and methods
The electroplating effluent used in the experiment was taken from a local electroplating unit. It consists of a dilution of a cyanide-free alkaline electroplating bath, where temperature and pH were determined using pH meter. A suitable volumewas taken before the dilution and was analyzed by volumetric method, using complexion III and indicator eriochrom Black T, buffer tampon pH 10. At the equivalence point, color changes from red to net blue. 1 ml complexionIII (0.05M) corresponding to 3,2685 mg of Zn[13].
Oxalispes-caprae were harvested fromthe field, stored and dried at room temperature. After complete drying of the plant, it wasgroundedto fine powder. Next,the obtained powder was washed by an appropriate solvent to eliminate chlorophyll. Finally,the particles which supposed to be poor of oxalic acid were separated by a mechanical method. Content of oxalate anion was analyzed by volumetric method with potassium permanganate 0,1Nat 80°C, in the presence of sulfuric acid 4N under magnetic stirring.At equivalence point, the solution became colorless. 1 mL KMnO4 0,1 N corresponds to 4,4 mg of C2O42-[2].The pH-metric titration of the raw and treated oxalis powders were carried out for the estimation of the oxalate anion and the free oxalic acid content and to confirm mechanic purification efficiency.
After dilution of the electroplating bath solution with demineralized water,Zn concentration metal content was 140 mg/L. Threeliters of this solution were prepared and stored for experiments. A volume of 100 mL was then taken in a beaker (250mL)and 10%suspension of treated oxalis powder(KITININ) was added gradually with agitation at a speed of 50 rpm at room temperature.After treatment,Znwas measured by Atomic Absorption Spectroscopy.
Results and discussion
The electroplating cyanide-free alkaline solution bath taken from a local electroplating unit was analyzed for the quantitative determination of Zn metal and sodium hydroxide contents. Zn was found to be 25 g/L and the concentration of NaOH rang of 125 g/L. A quantity of this concentrated solution was diluted by adding demineralized water to 140 mg/L of Zn metal and the final pH was 11,09.The residual concentration of cations in electroplating effluent can be taken into account apart from 50-100 mg/L up to 5-6 g/L according to reference 2.On the other hand, after washing the prepared oxalis powder (KITININ) with an appropriate solvent, the green color corresponding at chlorophyll molecules was successfully eliminated. After drying,the powder was yellow color and particles supposed to bepoor of oxalicacid were separated by mechanical method on basis of density. Content of oxalate anion in treated oxalis powder was analyzed by volumetric method with potassium permanganate, and concentration of 130 mg/g was obtained. A pH-metric titration with KOH(O,4N) of the raw and treated oxalis powder was carried out (figure 1).When free oxalic acid is titrated with a strong base (KOH), the titration curve has two equivalence points. These correspond to the removal of each successive proton from the diacid, and occur at pH 2.71 and pH 8.36.
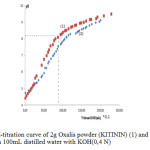 |
Figure 1:pH-titration curve of 2g Oxalis powder (KITININ) (1) and 2g raw oxalis powder (2) in 100mL distilled water with KOH(0,4 N) Click here to View Figure |
From the titration curves it is clear that the oxalic acid is in its monometallic form (mono potassium salt) as the initial pH corresponds to the end of the first half-neutralization. The pH of a suspension of 2 g of treated oxalis powder becomes more acidic than the crude oxalis powder,but the equivalent volume is almost the same. According to the equivalent volume around 9 mL, the concentration of monopotassium oxalate in the treated powder oxalis (KITININ) is estimated at 12%.The oxalic acid content in oxalis plant was usually about 16% of the dry weight according to reference 17.
Up on the addition of oxalis suspension,the flocks coalesced and settled down immediately and the Zn elimination was monitored with conductivity measurement.In effect, if the divalent Zn interacts with oxalic acid present in there soluble form and adsorbed by the plant tissues, it is compulsory that the conductivity decreases. The latter was decreased with added mass of oxalis powder, due to the mobilization of Zn as oxalates as shown in figure 2.The main part of heavy metal (Zn)was removed from the sludge, the suspensions were centrifuged at 3000 rpm for 5 min and the supernatant wast hen filtered for Zn analysis using absorption spectrometry (AAS). The results show about 98,56 % of zinc removal.
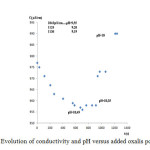 |
Figure 2: Evolution of conductivity and pH versus added oxalis powder mass Click here to View table |
In our experiment zinc was removed by precipitation as metal insoluble complex, and oxalis powder can be considered as a flocculent agent. Determining the effectiveness of ourbio-chelating agent for Zn removal from electroplating cyanide-free effluent has commonly been accomplished in one-step batch extractions at the laboratory-scale. Such extractions often encounter some limitations and difficulties:reverse reactions and delayed precipitation.
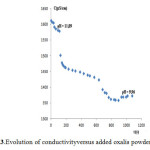 |
Figure 3: Evolution of conductivityversus added oxalis powder mass Click here to View Figure |
In a first test (Figure 2) the optimum dose has been exceeded in order to neutralize the effluent after zinc mobilization. But the addition of an excess of powder oxalis had a low effect on pH adjustment as the pH in the vicinity of the optimum dose was decreased from 11 to 10.49. By adding advantage of oxalis powder, the conductivity of the treated oxalis powder increased gradually to 1043, 1124 and 1136 µS/cm for a pH reduction only equal to 9.55, 9.26 and 9.19 respectively. Therefore a correction of the pH was necessary using a mineral acid, such as hydrochloric or sulfuric.
The effect of the pH on the removal of Znmust be examined because the waste waterfrom plating factory was of various pH values. In our study, effluent samples were used without pH adjusting. Usually, the optimum pH values for Zn precipitation range is 8-10.From the experimental figure 3 of conductivity versus oxalis powder dose, it was evident, that pH 10was optimum for complexation of Zn with oxalic acid and 5-6 g/L of KITININ is sufficient for the treatment of zinc electroplating cyanide-free effluent concentration ranging from 100 to 150 mg/L of Zn metal.
Acknowledgements
We Acknowledgement assistance from the AFAK CONTROL Laboratory, Oran-Algeria for AAS analysis
Conclusion
A simple and cost effective treatment procedure was proposed for the removal of zinc from zinc plating industry effluent using oxalis plant in its powder form. The most important thing is that the Zinc recovery ions process with 98,52% efficiency.The low zinc oxalate solubilityproduct value is an advantage for the treatment by complexation/flocculation/settling and filtration of waters with zinc ion content. Using this method, it is not only possible to remove the Zinc from the cyanide-free alkaline electroplating effluent but also to recover the transitional metal (Zn).Zinc oxalate adsorbed on organic plant oxalis powder could be turn into oxide. This work is an attempt to develop new bio chelating/flocculent agent for heavy metals removal from dilute solutions and to the reduction of zinc, chrome, lead etc to very low concentration less than 5ppm.
References
- Grebenyuk V.D., Sorokin G.V., Verbich S.V., Zhiginas L.H.,Linkov V.M., Linkov N.A.,Smit J.J. (1996) Combined sorptiontechnology of heavy metal regeneration from electroplating rinsewater. Water SA 22(4) 381-384.
- Gavris G., Cozma A., Ursu M-P, Petrehele A. (2009) Studies upon Zinc ion recovery from aqueusrezidualwastewater.Determination of the optimum conditions(II), Nonconventional Technology Review N°2m 32-35
- Mukesh Parmar1* and Lokendra Singh ThakurHeavy metal Cu, Niand Zn: Toxicity, Health Hazards andtheir removal techniques by low cost adsorbent: A short overview, international journal of plant, animal and environmenttal sciences, 3,3 2013. 143-157
- Xiaoyun Li, Xiuling Chen, Xiumin Cui (2012) Zinc chemical forms and organic acid exudation in non-heading Chinese cabbages under zinc stress Vol.3, No.4, 562-566 (2012)
- Bernard E.,Jimob A. (2013).Adsorption of Pb,Fe,Cu and Zn from industrial electroplating wastewater by orange peel activated carbon.Interntional journal of engineering and applied sciences 4,2,95-102.
- Sachin M. Kanawade, R.W. Gaikwad (2011) Removal of zinc ions from industrial effluent by using cork powder as adsorbent. International journal of chemical engineering and applications. 2, 3, 199-201
- Santhosh P., Sridevi A. (2013) A lab-scale study on reduction of heavy metals from electroplating effluent using conventional chemical precipitation. J. Environ. Res. Develop. 8,1,102-108.
- Nour T. Abdel-Ghani1 and Ghadir A. El-Chaghaby( 2014)Biosorption for metal ions removal from aqueous solutions: A review of recent studies.International Journal of Latest Research in Science and Technology. 3,1, 24-42.
- NafaˆaAdhoum∗, LotfiMonser, NizarBellakhal, Jamel-EddineBelgaiedTreatment of electroplating wastewater containing Cu2+, Zn2+and Cr(VI) by electrocoagulation. Journal of Hazardous Materials B112 (2004) 207–213
- Vishal Mishra(2014) Biosorption of zinc ion: a deep comprehension. appl. Water Sci. 1-22
- Adel A. Al –Hemiri, Heaven E. Mahmoud Minimization of Toxic Ions in Waste Water Using Emulsion Liquid Membrane Technique Iraqi Journal of chemical and Petroleum Engineering Vol.11 No.1 (March 2010) 11-19
- Yongabi K. A. (2009) Biogoagulants for water and waste water purificatio: a review International review ofchemical engineering 2,3,444-458
- Edward Budman(1995)Alkalinenoncyanide zinc plating , Metal Fnishing,60-64
- 2011 International Conference on Environment Science and Engineering IPCBEE vol.8 (2011) © (2011) IACSIT Press, SingaporeRemoval of heavy metals from a contaminated calcareous soil using oxalic andacetic acids as chelating agents S. Oustan, S. Heidari , M.R. Neyshabouri, A. Reyhanitabar, A. Bybordi
- Jong-Oh Kim, Yong-Woo Lee, Jinwook Chung(2013) The Role of Organic Acids in the Mobilization of Heavy Metals from Soil. KSCE Journal of Civil Engineering 17(7)1596-1602
- Henry Kqsqini,paul Thabo Kebana, AmiraliAlizadehSaghti, Kim Bolton (2013) Adsorption Characteristcs of Cobalt and Nickel on Oxalate-treated activated carbones in sulphate media. Wold Academy of Science, Engineering and Technology 76,707-721
- Adele Millerd, R. K. Morton, J. R. E. Wells (1963)Oxalic Acid Synthesis in Shoots of Oxalis pes-caprae (L.)Biochem.J. 86, 57

This work is licensed under a Creative Commons Attribution 4.0 International License.









