NMR and FTIR analysis of overheated cooking oil
Théoneste Muhizi*
University of Rwanda, School of Pure and Applied Science, Department of Chemistry, University avenue 117 Butare, Rwanda.
DOI : http://dx.doi.org/10.13005/ojc/300233
Article Received on :
Article Accepted on :
Article Published : 01 May 2014
In this study, we combined different physico-chemical methods to show the effect of overheating on the quality of golden fry cooking oil. Those methods include spectroscopic methods such us 1H-NMR, 13C-NMR and FTIR. Results showed that both overheating and excessive reuse of golden fry cooking oil seriously affected its quality by increasing its density, viscosity and different indexes like refractive, saponification, esters, peroxide indexes and also by decreasing its iodine index. Both NMR and FTIR spectroscopic methods confirmed these changes and indicated the presence of some carbonyl compound derivatives in overheated samples.
KEYWORDS:Overheating; cooking oil; physico-chemical analysis; NMR; FTIR
Download this article as:| Copy the following to cite this article: Muhizi T. Nmr and Ftir analysis of overheated cooking oil. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Muhizi T. Nmr and Ftir analysis of overheated cooking oil. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3229 |
INTRODUCTION
Oils are one of natural compounds widely used by human for both social and economical issues. Due to their various origins, oils are chemically different so that their uses are also variable. Food preparation, cosmetic industries and artisanal lubricants are some wide domains in which oils are used. Nowadays, oils are even largely needed to manufacture biodiesel, fuel with environmental friendly properties. In food preparation, cooking oils is used either for dressing, sautéing or frying food. Depending on what it is used for, cooking oil should be used differently to protect it against degradation. Its higher sensitivity to external factors such as heating, ultraviolet light, air and moisture may often cause its decomposition and leads to different problems in human body1-4. Nowadays, fried foods have become very popular to consumers due to their good flavour, colour and crispy texture3. However, during frying, not only a high temperature is applied on both oil and food, but also oil receives moisture from food leading to its rapid decomposition. Furthermore, frying is often conducted under air that accelerates oil degradation into different volatile and non volatile by-products with suspected toxicity. Thus the non volatile ones remain in the oil, change both physical and chemical properties and also negatively affect quality of foods. The presence of all these new elements in the oil undergoes further chemical reactions producing various compounds with affect its flavour stability, quality and texture. Finally, these suspected toxic compounds are absorbed with fried foods5 and this may constitute human intoxication risk. Carbonyls and free radicals, compounds with known toxicity6-8, are the major components of these. Carbonyl and free radical compounds are known as precursor of different diseases in human body including cancer, atherosclerosis, inflammatory joint disease, asthma, diabetes, senile dementia and degenerative eye diseases9. To avoid these different health risks, frying method should be carefully controlled and type of cooking oil well chosen5. Due to the ignorance of developing countries’ people on the associated risks, fried cooking oil is often reused more times. Therefore, it is the responsibility of scientists to work hard in informing and advising people on both best practices in cooking and also on the quality of oil to use and this in the aim to avoid human intoxication. In this study we verified the effect of overheating on the quality of golden fry oil prior to conclude on its efficacy to resist on overheating process.
EXPERIMENTAL
Sampling
Golden fry oil (Bidco Uganda Limited, Uganda) was sampled from the students’ restaurant of the University of Rwanda, Huye Campus, during frying potatoes. Before, sampling we were aware that cooks in this restaurant did not frequently discharge used oil but preferred to complete the remaining in fryers by the fresh one to make sufficient quantity for frying potatoes. For this reason, we started sampling with the first day of frying, directly after the beginning of the academic year where fryers didn’t contain any reused oil. Three samples named B, C and D, representing oils used for one, five and ten times respectively were collected. As reference, the unused oil was also sampled and named A. Before frying, temperature reached by oil was taken and noted. All samples were collected in opaque vials and kept at 0°C prior to be chemically analysed. They have been purified using decantation and filtration methods before further analysis.
Determination of physico chemical parameters
The density of samples was measured with a bottle pychnometer (Cole-Palmer) while the viscosity of samples was measured using a capillary viscosimeter (Cole-Palmer) using methods previously described10, 11. Refractive, saponification, iodide, acid, ester and peroxide values were determined as described in the previous scientific reports10-14.
FTIR, 1H NMR and 13C NMR analysis
FTIR spectra were recorded on Perkin-Elmer-Paragon 1000 PC spectrophotometer. Ten micro litres (10 μL) of sample is spread between two KBr pellet discs previously fabricated and then spectra were recorded between 400 and 4000 cm-1 using 50 scans at a resolution of 4.0 cm-1. 1H NMR and 13C NMR spectra were recorded at 300 MHz on Bruker Avance 300 spectrometer. Chemical shifts are given in ppm.
RESULTS AND DISCUSSION
In this study, golden fry oil reached a flash point of 186°C prior to fry potatoes. According to different authors, this temperature is sufficient to highly decompose many frying oils into highly reactive agents15-18. Considering that frying was conducted in non covered cooking utensils, air was in direct contact with both oil and fried potatoes. Consequently, in conjunction with oxygen and other substances released from potatoes, these reactive agents accelerate further degradation of the oil and lead to the formation of various by products with suspected side effects on human health. Degradation of golden fry oil during frying was preliminary remarked through different colours obtained from samples which varied from clear yellowish, yellow orangey, yellow orangey to red-orangey for samples A, B, C and D, respectively (Figure 1).
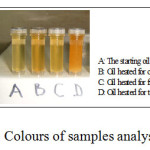 |
Figure 1: Colours of samples analysed. Click here to View Figure |
This preliminary observation on the degradation of golden fry oil was confirmed by physicochemical characteristics of different samples analysed (Table 1). The density, viscosity, different indexes including refractive, saponification, acid, oxidised acids, ester and peroxide values were increased with time of heating. These suggested a possible degradation of the starting oil (sample A). The density varied from 0.933 to 0.9443 for the samples A and D respectively, while the viscosity changed from 5.2 to 5.9 for the samples A and D respectively. The increasing of these two parameters from sample A to the overheated oil, sample D, may be in relationship, not only with chemical changes, especially the increasing of free fat acids in the oil, but also with some droplet released into the re-used oil10. Refractive index increased from 1.473 to 1.568 for samples A and D respectively, while iodide index decreased from 122 to 108 for A and D samples respectively. These changes may suggest a polymerisation or an oxidation of the starting oil during its heating and re-using12. Furthermore, increasing of both saponification and acid values may indicate a relationship between heating and formation of free fatty acids. Higher index values in the more heated oil may indicate that oil was hydrolysed during heating.
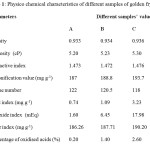 |
Table 1: Physico chemical characteristics of different samples of golden fry oil Click here to View table |
These results corroborate with literature which reported that hydrolysis of triglycerides should be accompanied by the increasing of free fatty acids in the oil10, 19. Iodide index was decreased with time of heating and re-using and this was closely related to the decreasing number of double bonds initially composing unused oil. This phenomenon may be due to the oxidation process of olefin bonds conducting to the formation of polar compounds in the re-used oil10. The increasing of acid, peroxide, ester, oxidised acids indexes in the oil confirmed and supported this suggestion.
FTIR, 1H NMR and 13C NMR characteristics
In Fourier Transform Infrared (FTIR) spectroscopy, four samples A, B, C and D indicated big ressemblances at short waveleghts. However at wavelenghts of 1653 and 3472 cm-1 only sample A absorbed while there was no absorbance for samples B, C and D. Furthermore, at 3610 cm-1, samples B, C and D largely absorbed while sample A did not (Figure 2).
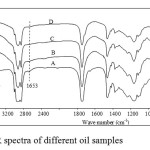 |
Figure 2: FTIR spectra of different oil samples Click here to View Figure |
Different FTIR bands of absorbance were attributed as shown in table 2.
 |
Table 2: Attribution of some absorption bands from oil samples Click here to View table |
In FTIR spectra (Figure 2), sample A, presented an absorption band at 3472 cm-1 indicating C-H for alkenes elongation. This band was confirmed by other absorption band remarked at 1653 cm-1 indicating C=C elongation. These two absorption bands were not observed from samples B, C and D. Furthermore, heated oils (samples B, C and D) presented absorption band at 3610 cm-1 which band was not detected from sample A. These differences remarked between unused oil and heated samples in IR absorption may indicate hydrolysis of triglyceride: break down of ester bonds actually found in sample A and release of glycerol, mono/di- acyl glycerol and fat acid. The later compounds are found in samples B, C and D. Glycerol, mono/di- acyl glycerol contain free OH-groups and are able to absorb around 3610 cm-1 as remarked in IR spectra20.
Findings from FTIR spectroscopy were confirmed by both proton and carbon nuclear magnetic resonance (1H NMR and 13C NMR) spectroscopy.Signals of 1H NMR spectra of both samples A and D (Figures 3 and 4) indicated that golden fry oil is mainly composed by a triglyceride of linoleic acid. 1H NMR pics were attributed to different protons of this triglyceride as shown in the table 3. These results from NMR spectroscopy confirmed those from other methods previously used.
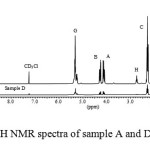 |
Figure 3: 1H NMR spectra of sample A and D Click here to View Figure |
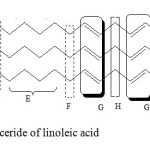 |
Figure 4: Triglyceride of linoleic acid Click here to View Figure |
Table 3: Chemical shifts of different protons found in samples A and D
 |
Table 3: Chemical shifts of different protons found in samples A and D Click here to View table |
A comparison done between 1H NMR of samples A and D (Figure 3) indicated that signal at 2.75 ppm (zone H) was absent from sample D. This signal represents two protons of C-H separating two double bonds of linoleic acid as previously reported21, 22. Its absence from sample D confirmed the break down of double bonds of linoleic acid leading to the formation of other products. Further analysis of 1H NMR spectra, at chemical shifts varying between 7.2 and 10.4 ppm where signals of carbonyl group may be appeared, indicated clear differences between heated oils and unused one (Figure 5). In addition, comparison done between samples A and D at chemical shifts of 5.6-8.4 ppm indicated also new signals in sample D (Figure 5). Zoomed zones showed new signals from all overheated and reused oils (samples B, C and D) at dH 9.4-9.8 ppm and at dH 5.8-7.2 ppm and this comparatively to the sample A. These signals may indicate formation of aldehydic or acidic group of compounds in these overheated oils20.
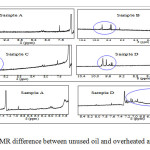 |
Figure 5: 1H NMR difference between unused oil and overheated and reused oils Click here to View Figure |
In 13C NMR spectra and comparatively to the spectrum of sample A, new signals were detected from overheated samples around the chemical shifts of 170, 174.5 and 181 ppm for sample D, 179 ppm for samples B, C and D, and 189.5 ppm for samples C and D. These 13C NMR findings corroborated with results from 1H NMR and indicated the presence of carbonyl groups such as aldehydic and acidic compounds in analysed samples20. Sample D which was from the more reused oil, was the more decomposed, it presented new broad signals at chemical shifts varying between 5.8 and 7.2 ppm in 1H NMR and also new signals at 170, 174.5 and 181 ppm in 13C NMR, which signals were absent from other samples. These polar compounds may be formed during heating where oxidation of double bonds and hydrolysis of ester bonds in the overheated samples took place. According to some authors, during heating, water, vapours and oxygen induced different chemical reactions in the fried oil, and these lead to the formation of different chemical oxidants9, 5. As nucleophile compound, water is able to attack ester bond of triglyceride and lead to the production of a mixture of free fatty acids, glycerol and mono-, di- and acyl- glycerol in the used oil. Furthermore, times of re-using cooking oil are also closely in relationship with its decomposition23, 24. Through spectroscopic methods, this study showed that after one time of use, golden fry oil was degraded. This higher degradation under heating may be due to the presence of linoleic acid in the oil since it was reported that oils mainly composed by linoleic acids are more easily polymerised during frying than those containing oleic acids23-25. The presence of carbonyl groups in overheated oil may constitute a serious risk to the human body. It was reported that aldehydes are potentially toxic substances with possibility to induce different cancers when consumed26-28. Furthermore, it is known that asparagine and reducing sugars are relatively in high amount in potatoes, and that both compounds are the main precursors of acrylamide, which is formed during frying, baking, grilling or toasting carbohydrates rich foods19, 29, 30. For this reason, signal observed at 181 ppm in 13C NMR spectrum of sample D may represent this compound. However further studies are required to well analyse and illustrate all by-products formed for conclusion. Note that acrylamide is considered as neurotoxic and classified as probably carcinogenic to humans by the International Agency for Research on Cancer 19, 29, 30.
CONCLUSION
All cooking oils, especially those used during frying should be discharged after one time of use to avoid consuming toxic compounds together with food. This study showed that heating oil at higher temperature for only one time is sufficient to break down its double bonds, even with possibility to lead to the formation of harmful compounds. Consequently, fried foods should be consumed with care and in moderate manner while golden fry oil assessed in this study may not be preferred as frying oil but can be used for other cooking services that don’t require high temperature.
Acknowledgment
We are very grateful to the University of Rwanda, especially the Research Commission to allow realization of this research and Mrs Pacifique Umubyeyi for technical assistance.
References
- Brown W., Rogers E., General Organic Biochemistry, Williard Grant Press, Boston, 811 p. (1980).
- Soriguer F., Rojo-Martinez G., Dobarganes M. C., Almeida J. M. G., Esteva I., Bertran M., De Adana M.S.R., Tinahones F., Gomez-Zumaquero J. M., Garcia-Fuentes E., Gonzalez-Romero S., Am. J. Clin. Nutr. 78: 1092 (2003).
- Boskou D., Salta F. N., Chiou A., Troullidou E., Andrikopoulos N. K., Eur. J. Lip. Sci. Tech. 108: 109 (2006).
- Mestdagh F., Castellan P., De Meulenaer B., Van Peteghem C., J. Agric. Food Chem 56 (15): 6141 (2008).
- Choe E., Min D. B., J. Food Sci. 72(5): 77 (2007).
- Stohs S. J., J. Basic Clin. Physiol. Pharmacol., 6 (3-4): 205 (1995).Find all citations by this author (default). Or filter your current search
- Dellinger B., Pryor W. A., Cueto R., Squadrito G. L., Hegde V., Deutsch W. A., Chem. Res. Toxicol. 14(10): 1371 (2001).Find all citations in this journal (default).
- O’Brien P.J., Siraki A. G., Shangari N., Crit. Rev. Toxicol. 35(7): 609 (2005)
- Bagchi K., Puri S., East. Mediterr. Health J. 4(2): 350 (1998).
- Noirfalise A., Foussin A., Méthodes d’analyse des substances alimentaires. 4ème édition, Université de Liège, 282 p. (1981).
- Audigie C., Figarella J., Zonszain F., Manipulations d’analyse biochimique, Doin Editeurs, Paris, 274 p. (1982).
- Gautier J. A., Pellerin F., Renault J., Fiches techniques d’analyse bromatologique. Société d’édition d’enseignement supérieure, Imp. Jouve, Paris, 230 p. (1961).
- OMS, Méthodes générales d’analyse, Volume I, 3ème édition, Pharmacopée internationale, Genève, 860 p. (1980).
- Solomon S., General Organic and Biological Chemistry, McGraw-hill Inc., USA, 845 p. (1987).
- Lin J. M., Liou S. J., Bull. Environ. Contam. Toxicol. 64: 817 (2000).
- Ndjouenkeu R., Ngassoum M., J. Food Eng., 52(2): 121 (2002).
- Gertz C., Klostermann S., Eur. J. Lip. Sci. Tech. 104: 762 (2002).
- Nglum C., Wehling R. L., Cuppett S. L., J. Agric. Food Chem 55: 593 (2007).
- Mestdagh F., De Meulenaer B., Van Peteghem C., J. Agric.Food Chem 100: 1153 (2007).
- Silverstein M. R., Clayton B. G., Morill T. C., Larue E., Schanck A. Identification spectrométrique des composés organiques. De Boeck Université, Paris, 1998; 432.
- Ogawa J., Mastumura K., Kishino S., Omura Y., Shimizu S., Appl. Envir. Microbiol., 67(3): 1246 (2001).
- Ying C., Lin Y., Hong-Li G., Jing-Nan C., Zhen-Yu C., Qiu-Shi R., Chem. Phys. Lipids 145: 128 (2007).
- Takeoka G. R., Full G. H., Dao L. T., J. Agric. Food Chem 50: 4998 (1997).
- Tompkins C., Perkins E. G., J. American Oil Chemists’ Society 77: 223 ( 2000).
- Bastida S., Sanchez-Muniz F. J., Food Sci. Technol. 7: 15 (2001).
- Wang R., Nakajima T., Kawamoto T., Honma T., Drug Metab. Dispo. 30 (1): 69 (2002).
- Yokoyama T., Yokoyama A., Kato H., Tsujinaka T., Mute M., Omori T., Haneda T., Kumagai Y., Igaki H., Yokoyama M., Watanabe H., Yoshimizu H., Cancer Epidemiol. Biomarkers Prevent. 12 (1): 1227 (2003).
- Boccia S., Hashibe M., Galli P., De Feo E., Asakage T., Hashimoto T., Hiraki A., Katoh T., Nomura T., Yokoyama A., van Duijn C., Ricciardi G., Boffetta P., Cancer Epidemiol. Biomarkers Prevent. 18 (1): 248 (2009).
- Pedreschi F., Moyano P., Kaack K., Granby K., Food Res. Int. 38: 1 (2005).
- IARC, Acrylamide, International Agency for Research on Cancer, Lyon, France, 1994.

This work is licensed under a Creative Commons Attribution 4.0 International License.









