Microwave-Assisted Synthesis of Potent Antimicrobial Agents of Flavanone Derivatives
Abdullah S. Albogami1*, Hamad Z. Alkhathlan2, Tamer S. Saleh1 And Ahmed M. Elazzazy3
1Department of Chemistry, Faculty of Science, King Abdulaziz University, North Jeddah, P.O Box 80203 Jeddah 21589, Saudi Arabia
2Department of Chemistry, College of Science, King Saud University, P.O.Box 2455 Riyadh - 11451, Saudi Arabia
3Biological Sciences Department, Faculty of Science, North Jeddah, King Abdulaziz University, P.O. Box 80327, Jeddah 21454, Saudi Arabia
DOI : http://dx.doi.org/10.13005/ojc/300205
Article Received on :
Article Accepted on :
Article Published : 29 Apr 2014
The currently available antimicrobial drugs suffer from toxicity, interactions with other drugs, insufficient pharmacokinetic properties, and the development of resistance. Thus, development of new antimicrobial agents with optimum pharmacokinetics and low toxicity is important. In this study, a series of flavanone, hydrazone derivatives have been prepared from flavanone under microwave irradiation after a very short reaction time (1-2 min.) with good yields. The structures of the synthesized compounds were elucidated using various spectroscopic methods. The screening of the synthesized compounds for antimicrobial activity was performed against Staphylococcus aureus, Escherichia coli, C. Albicans and Aspergillus niger. Some of the synthesized compounds show potent anti microbial activity.
KEYWORDS:Microwave irradiation; Flavanone; Hydrazone; Isoniazide; Antimicrobial activity.
Download this article as:| Copy the following to cite this article: Albogami A. S, Alkhathlan H. Z, Saleh T. S, Elazzazy A. M. Microwave-Assisted Synthesis of Potent Antimicrobial Agents of Flavanone Derivatives. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Albogami A. S, Alkhathlan H. Z, Saleh T. S, Elazzazy A. M. Microwave-Assisted Synthesis of Potent Antimicrobial Agents of Flavanone Derivatives. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3192 |
INTRODUCTION
Flavanones, which exhibited broad spectrum of biological activities, have long attracted the interest of chemists. The most commonly reported biological activities include neuron protection, anti-tumor, anti-metastasis, anti-microbial, anti-oxidant, anti-inflammatory, and anti-viral activities1-8. Flavanones, which have chemical structures embedded with a 2-aryl chroman-4-one skeleton, are widely distributed in plants9 and are available from synthetic sources. The major and most commonly reported synthetic methods for flavanones 4 usually involve the Claisen Schmidt reaction of o-hydroxyacetophenones 1 with benzaldehydes 2 to produce chalcone intermediates 3 using various catalysts10-14, followed by cyclization with various bases15, acids16, or other materials (Scheme 1)17.
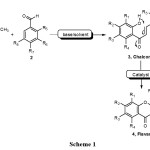 |
Scheme 1 Click here to View Scheme |
Recently, we reported the simple and efficient microwave-assisted synthesis of 2′-hydroxychalcones and flavanones in one step with high yield and no side products14 highlighting the role of different substituent in directing the reaction to pure chalcone or flavanone product (Figure 1).
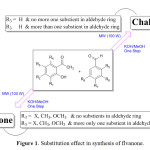 |
Figure 1: Substitution effect in synthesis of flvanone. Click here to View Figure |
The use of microwave irradiation in organic synthesis has become increasingly popular within the pharmaceutical and academic areas, as a new technology enabling drug discovery and development18,19. By taking advantage of this efficient source of energy (enhanced reaction rates, high yields, improved purity, ease of work up after the reaction and eco-friendly reaction conditions relative to conventional methods), compound libraries for lead generation and optimization can be assembled in a fraction of the time required by classical thermal methods.
Prompted by these observations and the importance of flavanones, we report the microwave-assisted synthesis of new flavanone derivatives and the evaluation of their antimicrobial activity.
EXPERIMENTAL
Materials and methods
All materials and reagents of the best available quality were purchased from commercial sources and used without further purification. Melting points are uncorrected and were determined on Gallenkamp-melting point apparatus. NMR spectra were recorded on JEOL ECP 400 (400 MHz) in CDCl3 and expressed as δ in ppm. Mass spectra were recorded on Shimadzu QP-5050A GC/MS system. Microwave experiments were carried out using CEM MARS synthatorTM microwave apparatus. TLC was performed on (TLC plates silica gel 60F245 pre-coated 20×20 cm layer thickness 0.25 mm). Elemental analyses were carried out on EuroVector instrument C, H, N, S analyzer EA3000 Series. Microwave experiments were carried out using CEM MARS synthatorTM microwave apparatus with temperature control for MW experiments using IR sensor. Flavanone 4a-d14 was prepared according to the reported literature.
Typical procedure for synthesis of flavanone derivatives 5a-d and 10a-d
An equimolar amount of flavanone 4a-d and phenyl hydrazine or isoniazide were mixed in a process vial in presence of 1 ml acetic acid and irradiated with microwave (Power 300 watt) for 1-2 min (as examined by TLC), after reaction completion the precipitate was filtered and washed with ethanol and the products (81-92% yield) were crystallized from ethanol. The synthesized compounds with their physical data are listed below.
1-(6-Bromo-2-phenylchroman-4-ylidene)-2-phenylhydrazine.
m.p. 112-114 oC, yield 81%; IR (KBr): 3327 (NH), 1605 (C=N), cm-1; 1H NMR (400 MHz, CDCl3) δ: 2.76 (dd, 1H, J = 16.8, 5.1 Hz, H3a), 3.34 (dd, 1H, J = 2.9, 16.8 Hz, H3b), 5.25 (dd, 1H, J = 2.9, 11.7 Hz, H2), 7.26-7.85 (m, 13H, Ar-H), 9.56 (s, 1H, NH, D2O-exchangeable), 13C NMR (100 MHz, CDCl3) δ: 39.9, 77.2, 112.5, 114.9, 116.6, 121.7, 124.3, 127.0, 127.6, 128.0, 128.8, 129.7, 134.1, 139.9, 143.1, 147.9, 155.1. MS (m/z): 392 (M+). (Found: C, 64.38; H, 4.28; N, 6.95 C21H17BrN2O Calc. C, 64.13; H, 4.36; N, 7.12%).
1-(6-Chloro-2-phenylchroman-4-ylidene)-2-phenylhydrazine. 5b
m.p. 194-195 oC, yield 88%; IR (KBr): 3323 (NH), 1599 (C=N), cm-1; 1H NMR (400 MHz, CDCl3) δ: 2.77 (dd, 1H, J = 16.8, 4.4 Hz, H3a), 3.36 (dd, 1H, J = 4.4, 16.8 Hz, H3b), 5.22 (dd, 1H, J = 2.2, 11.7 Hz, H2), 7.19-7.77 (m, 13H, Ar-H), 9.55 (s, 1H, NH, D2O-exchangeable), 13C NMR (100 MHz, CDCl3) δ: 39.7, 77.2, 112.1, 114.2, 119.8, 121.00, 125.1, 126.1, 127.5, 128.0, 128.9, 129.9, 134.0, 139.5, 143.0, 145.2, 154.7. MS (m/z): 348 (M+). (Found: C, 72.64; H, 4.78; N, 7.83; C21H17ClN2O Calc. C, 72.31; H, 4.91; N, 8.03%).
1-(6-Methoxy-2-phenylchroman-4-ylidene)-2-phenylhydrazine. 5c
m.p. 174 oC, yield 81%; IR (KBr): 3298 (NH), 1605 (C=N), cm-1; 1H NMR (400 MHz, CDCl3) δ: 2.72 (dd, 1H, J = 16.9, 5.1 Hz, H3a), 3.33 (dd, 1H, J = 16.9, 2.9 Hz, H3b), 3.83 (s, 3H, OMe), 5.16 (dd, 1H, J = 2.9, 11.7 Hz, H2), 7.21-7.88 (m, 13H, Ar-H), 9.42 (s, 1H, NH, D2O-exchangeable), 13C NMR (100 MHz, CDCl3) δ: 39.7, 50.1, 77.1, 112.3, 115.0, 116.5, 119.0, 121.6, 126.5, 127.8, 128.1, 129.4, 139.1, 143.0, 147.2, 148.9, 154.4. MS (m/z): 344 (M+). (Found: C, 77.02; H, 5.73; N, 7.95; C22H20N2O2 Calc. C, 76.72; H, 5.85; N, 8.13%).
1-(2-(4-Chlorophenyl)-6-methoxychroman-4-ylidene)-2-phenylhydrazine. 5d
m.p. 133-135 oC, yield 82%; IR (KBr): 3322 (NH), 1602 (C=N), cm-1; 1H NMR (400 MHz, CDCl3) δ: 2.71 (dd, 1H, J = 16.8, 4.5 Hz, H3a), 3.35 (dd, 1H, J = 2.9, 17.6 Hz, H3b), 3.81 (s, 3H, OMe), 5.20 (dd, 1H, J = 2.9, 11.8 Hz, H2), 7.20-7.78 (m, 12H, Ar-H), 9.44 (s, 1H, NH, D2O-exchangeable), 13C NMR (100 MHz, CDCl3) δ: 39.9, 51.0, 76.3, 112.2, 114.9, 116.5, 119.1, 121.0, 126.2, 129.0, 129.9, 134.0, 136.6, 143.2, 147.2, 149.5, 154.5. MS (m/z): 378 (M+). (Found: C, 70.08; H, 4.92; N, 7.19; C22H19ClN2O2 Calc. C, 69.75; H, 5.05; N, 7.39%).
4-(2-(6-bromo-2-phenylchroman-4-ylidene)hydrazinyl)pyridine. 10a
m.p. 200-202 oC, yield 84%; IR (KBr): 3365 (NH), 1690 (C=O), 1598 (C=N), cm-1; 1H NMR (400 MHz, DMSO) δ: 2.77 (dd, 1H, J = 17.6, 5.1 Hz, H3a), 3.38 (dd, 1H, J = 16.8, 2.9 Hz, H3b), 5.25 (dd, 1H, J = 2.9, 11.7 Hz, H2), 6.92-7.88 (m, 12H, Ar-H and Pyridine-H), 9.56 (s, 1H, NH, D2O-exchangeable), 13C NMR (100 MHz, DMSO) δ: 39.6, 77.2, 113.4, 114.1, 114.8, 119.5, 122.7, 126.5, 127.6, 128.0, 133.0, 134.0, 139.1, 140.1, 145.9, 154.6, 160.2. MS (m/z): 421 (M+). (Found: C, 60.03; H, 3.69; N, 9.78; C21H16BrN3O2 Calc. C, 59.73; H, 3.82; N, 9.95%).
4-(2-(6-chloro-2-phenylchroman-4-ylidene)hydrazinyl)pyridine. 10b
m.p. 194-195 oC, yield 92%; IR (KBr): 3359 (NH), 1697(C=O), 1605 (C=N), cm-1; 1H NMR (400 MHz, CDCl3) δ: 2.75 (dd, 1H, J = 16.8, 5.1 Hz, H3a), 3.39 (dd, 1H, J = 17.6, 4.4 Hz, H3b), 5.29 (dd, 1H, J = 2.9, 12.4 Hz, H2), 6.89-7.91 (m, 12H, Ar-H and Pyridine-H), 9.56 (s, 1H, NH, D2O-exchangeable), 13C NMR (100 MHz, CDCl3) δ: 39.6, 77.2, 113.5, 114.0, 117.1, 122.9, 126.0, 127.6, 127.9, 128.5, 128.7, 132.0, 138.2, 139.9, 145.9, 154.6, 159.2. MS (m/z): 377 (M+). (Found: C, 66.99; H, 4.18; N, 10.98; C21H16ClN3O2 Calc. C, 66.76; H, 4.27; N, 11.12%).
4-(2-(6-Methoxy-2-phenylchroman-4-ylidene)hydrazinyl)pyridine. 10c
m.p. 144-145 oC, yield 88%; IR (KBr): 3361 (NH), 1691 (C=O), 1601(C=N), cm-1; 1H NMR (400 MHz, CDCl3) δ: 2.73 (dd, 1H, J = 16.8, 4.4 Hz, H3a), 3.36 (dd, 1H, J = 2.9, 16.8 Hz, H3b), 3.77 (s, 3H, OMe), 5.18 (dd, 1H, J = 2.9, 11.7 Hz, H2), 7.01-7.84 (m, 12H, Ar-H), 9.45 (s, 1H, NH, D2O-exchangeable), 13C NMR (100 MHz, CDCl3) δ: 39.8, 50.8, 77.1, 112.2, 114.7, 116.2, 117.0, 117.3, 121.9, 126.9, 127.1, 128.0, 138.0, 139.6, 147.1, 150.4, 154.4, 160.0. MS (m/z): 373 (M+). (Found: C, 70.98; H, 5.07; N, 11.09; C22H19N3O3 Calc. C, 70.76; H, 5.13; N, 11.25%).
4-(2-(2-(4-chlorophenyl)-6-methoxychroman-4-ylidene)hydrazinyl)pyridine. 10d
m.p. 124-126 oC, yield 86%; IR (KBr): 3359 (NH), 1690 (C=O), 1602 (C=N), cm-1; 1H NMR (400 MHz, CDCl3) δ: 2.71 (dd, 1H, J = 16.8, 4.4 Hz, H3a), 3.36(dd, 1H, J = 2.9, 16.8 Hz, H3b),3.71 (s, 3H, OMe), 5.20 (dd, 1H, J = 2.9, 12.4 Hz, H2), 6.89-7.92 (m, 11H, Ar-H), 9.45 (s, 1H, NH, D2O-exchangeable), 13C NMR (100 MHz, CDCl3) δ: 39.4, 51.0, 76.3, 112.8, 114.0, 116.1, 117.2, 117.9, 122.0, 126.2, 128.7, 134.1, 135.2, 139.7, 146.8, 150.2, 154.5, 160.0. MS (m/z): 407 (M+). (Found: C, 65.07; H, 4.33; N, 10.14. C22H18ClN3O3 Calc. C, 64.79; H, 4.45; N, 10.30 %).
Antimicrobial activity
The antimicrobial activity was investigated on the newly green synthesized Hydrazon, Flavanone and Iso-niazide compounds. The antimicrobial profile was evaluated by measuring the inhibitory effects and the potency of such compounds against Gram positive, Gram negative bacteria and fungi using the agar diffusion technique29.
The bacterial strains Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) were cultured on nutrient agar. The fungal strains Candida albicans (C. albicans) was maintained on Yeast malt extracts medium (YM), while Aspergillus niger (A. niger) was maintained on Czapeck Dox medium. All the four strains were used against the tested compounds for antimicrobial evaluation.
Suspension of the above mentioned bacterial strains was prepared by inoculating fresh stock cultures into separate broth tubes, each containing 7 ml of nutrient broth. The inoculated tubes were incubated at 27 ± 2°C for 24 h.
Solutions of the tested compounds and reference drugs were prepared by dissolving 0.5 g of the compound in chloroform (5 ml).
RESULTS AND DISCUSSION
As the condensation reactions of amines and hydrazines with ketones are usually catalysed by acids or bases, the synthetic route to phenylhydrazone derivatives 5a-d is illustrated in Scheme 2. The starting flavanone derivatives 4a-d were prepared from the corresponding 2′-hydroxyacetophenones according to the literature method14. The synthesis of phenylhydrazone derivatives 5a-d was attempted by the reaction of flavanone 4a-d with phenylhydrazine under microwave irradiation in the presence of 1 ml of acetic acid, which resulted in good to excellent yield of pure phenylhydrazone products in very short reaction times (1-2 min.). The synthetic route to phenylhydrazone derivatives 5a-d is illustrated in Scheme 2.
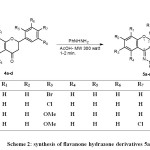 |
Scheme 2: synthesis of flavanone hydrazone derivatives 5a-d |
Theoretically, the reactivity of flavanone derivatives toward N-nucleophiles, such as substituted hydrazine, is related to two sites: the carbonyl group and C-2 position. The attack of hydrazine to the C-2 position of a chroman ring could result in ring opening and subsequent pyrazoline formation. Previously, the reaction of flavanone derivatives with substituted hydrazines has been investigated under different conditions20.21. Kállay et al. have reported that flavanone hydrazones are predominantly obtained under acidic conditions while alkaline conditions give the pyrazolines and the 2-hydroxychalcone derivatives due to the ring cleavage of the hetero ring of chromanone20. Similarly, in our experiment with flavanone 4a-d, the hydrazone derivatives were predominantly obtained under mild acidic conditions, Scheme 3.
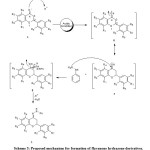 |
Scheme 3: Proposed mechanism for formation of flavanone hydrazone derivatives. Click here to View Scheme |
The structures of compounds 5a-d were characterized by IR, 1H NMR and mass spectroscopy as well as elemental analysis. For example, the IR spectra of compound 5a did not show any absorption due to the presence of C=O groups, and a broad band was observed at 3298-3327 cm-1 due to the presence of a N−H bond. The 1H NMR spectrum of 5a showed a singlet D2O exchangeable signal at d 9.56 for NH; three doublets of doublet signals at d 2.76 (J = 16.84, 5.12 Hz), 3.34 (J = 2.92, 16.84 Hz) and 5.25 (J = 2.96, 11.76 Hz) for H-3a, H-3b and H-2 of the chroman ring, respectively; and a multiplet of aromatic protons at d 7.26-7.85 ppm.
In a similar manner, the isoniazid (isonicotinylhydrazine) (9) reacted with flavanone derivatives 10a-d under microwave irradiation to give the corresponding hydrazone derivatives, Scheme 4.
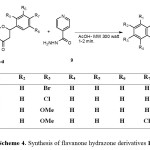 |
Scheme 4. Synthesis of flavanone hydrazone derivatives 10a-d. |
The structure of flavanone derivatives 10a-d was assigned based on their elemental analyses and spectral data. For example, the 1H NMR spectrum of compound 10a revealed a singlet D2O exchangeable signal at d 9.56 for NH; three doublets of doublet signals at d 2.77 (J = 17.60, 5.16 Hz), 3.38 (J = 2.92, 16.84 Hz) and 5.25 (J = 2.96, 11.76 Hz) for H-3a, H-3b and H-2 of the chroman ring, respectively; and a multiplet of aromatic protons at d 6.92-7.88 ppm. The mass spectrum of the same compound revealed a peak corresponding to its molecular ion at m/z 421.
It was found that microwave irradiation has a beneficial effect on the synthesis of flavanone derivatives. The above reactions took less than 5 min., and provided excellent yields.
Antimicrobial activity
The results in table 1 showed that flavanone compounds were the most potent among the three classes (flavanone, hydrazone and isoniazide) in terms of antibacterial activity. The highest inhibition zone against E. coli was detected by flavanone compound 4a followed by 4c, while, 4d was the most potent flavanone compound against S. aureus. Compounds 5d, 5c and 5b of hydrazone were the most potent and exhibited the highest inhibition zone against E. coli while, 5b was the most potent compound against S. aureus. The isoniazide 10c and 10b were the most potent compounds against E. coli and the 10b isoniazide was the most potent compound against S. aureus.
The results in table 2 revealed that hydrazone compounds were the most potent among the three classes (flavanone, hydrazone and isoniazide) in terms of antifungal activity. None of the flavanone compounds exhibited potency against C. albicans and A. niger. Compounds 5b and 5d of hydrazone were the most potent and exhibited high inhibition zone against C. albicans whereas 5c and 5d were the most potent against A. niger. Isoniazide 10a and 10b compounds were the most potent compounds against C. albicans and none of the isoniazide compounds had any effect against A. niger.
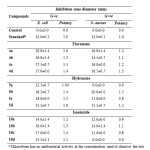 |
Table 1: Antibacterial activity of test compounds and reference druga Click here to View table |
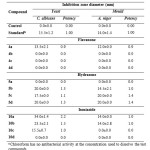 |
Table 2 : Antifungal activity of test compounds and reference druga Click here to View table |
Several recent reviews and studies have reported the antimicrobial efficacy of flavanone molecules22-24. The antimicrobial effectiveness of flavonoids is due to their abilities to form complexes with both extracellular and soluble proteins as well as bacterial membranes25,26. The penetration into the cell and the maintenance of intracellular concentrations in infecting species becomes a critical concern for the development of flavonoids as the next generation of antibacterial/antifungal agents.
Hydrazone-type compounds containing azomethine constitute an important class of compounds for new drug development. It is well known that the hydrazone group plays an important role in antimicrobial activity27. It has been claimed that a number of hydrazide-hydrazone derivatives possess interesting antibacterial and antifungal activities28.
 |
GRAPHICAL ABSTRACT |
CONCLUSIONS
A series of flavanone hydrazone derivatives have been prepared from flavanone under microwave irradiation in very short reaction time (1-2 min.) and good yields. Some synthesized compounds show potent anti-microbial activity.
REFERENCES
- Vauzour D., Vafeiadou K., Rice-Evans C., Williams R. J. and Spencer J. P. E., J. Neurochem., 103: 1355-1367 (2007).
- Agarwal C., Tyagi A., Kaur M. and Agarwal R., Carcinogenesis, 28: 1463-1470 (2007).
- Cabrera M., Simoens M., Falchi G., Lavaggi M. L., Piro O. E., Castellano E. E., Vidal A., Azqueta A., Monge A., Lopez de Cerain A., Sagrera G., Seoane G., Cerecetto H. and Gonzalez M., Bioorg. Med. Chem., 15: 3356-3367 (2007).
- Hsiao Y. C., Kuo W. H., Chen P. N., Chang H. R., Lin T. H., Yang W. E., Hsieh Y. S. and Chu S. C., Chem.-Biol. Interact., 167: 193-206 (2007).
- Ward F. E., Garling D. L. Buckler R. T. Lawler D. M. and Cummings D. P., J. Med. Chem., 24: 1073-1077 (1981).
- Choi Y. J., Lee M. K., Lee Y. J., Jeong Y. J., Yoon Park J. H., Sung L. S. and Kang Y. H., J. Med. Food, 7: 408-416 (2004).
- Njamen D., Mbafor J. T., Fomum Z. T., Kamanyi A., Mbanya J. C., Recio M. C., Giner R. M., Manez S. J. and Rios L. Planta Med., 70: 104-107 (2004).
- Paredes A., Alzuru M. and Mendez J., Biol. Pharm. Bull., 26: 108-109 (2003).
- Reddy M. V. B., Kishore P. H., Rao C. V., Gunasekar D., Caux C. and Bodo B., J. Nat. Prod., 66: 295-297 (2003).
- Wang X. and Cheng S., Catal. Commun., 7: 689-695 (2006).
- Drexler M. T. and Amiridis M. D. 214: 136-145 (2003).
- Chandrasekhar S., Vijeender K. and Reddy K. V., Tetrahedron Lett., 46: 6991-6993 (2005).
- Choudary B. M., Ranganath K. V. S., Yadav J. and Kantam M. L., Tetrahedron Lett., 46: 1369-1371 (2005).
- Albogami A. S., Karama U., Mousa A. A., Khan M. S., Al-Mazroa A. and Alkhathlan H. Z. Oriental J. Chem., 28: 619-626 (2012).
- Coetzee J., Mciteka L., Malan E., Ferreira D., Phytochemistry, 52: 737-743 (1999).
- Adams J. H., J. Org. Chem., 32: 3992-3998 (1967).
- Climent M. J., Garcia H., Iborra S. Miranda M. A. and Primo, J., Heterocycles, 29: 115-121 (1989).
- Lidstrom P., Tierney J., Wathcy B., Wcstman J., Tetrahedron, 57: 9225-9283 (2001).
- Stadler A., Yousefi B. H., Dallinger D., Walla P., der Eycken E. V., Kaval N. and Kappe, C. O., Org. Proc. Res. Dev., 7: 707-716 (2003).
- Kállay F., Janzsó G. and Koczor I., Tetrahedron, 23: 4317-4321 (1967).
- Léavai A. J. Heterocyclic Chem., 39: 1-13 (2002).
- Sobolev V. S. Neff S. A. and Gloer J. B., J. Agric. Food Chem., 57: 62-68 (2009).
- Abdel Ghani S. B., Weaver L., Zidan Z. H., Ali, H. M. W. and Keevil C., Bioorg Med Chem Lett., 18: 518-522 (2008).
- Nowakowska Z., Eur. J. Med. Chem., 42: 125-137 (2007).
- Cowan M. M., Clin. Microbiol. Rev., 12: 564-582 (1999).
- Fowler Z. L., Baron C. M., Panepinto J. C. and Koffas M. A., Yeast, 28: 181-188 (2011).
- Abdel-Fattah M. E., Salem E. E. and Mahmoud M. A., Ind. J. Het. Chem., 10: 121-128 (2000).
- Vicini P., Zani F., Cozzini P. and Doytchinova I., Eur. J. Med. Chem., 37: 553-564 (2000).

This work is licensed under a Creative Commons Attribution 4.0 International License.









