Linseed (Linum usitatissimum L.) as a biocatalyst for reduction of nitroaromatic compounds
Leonardo C. Tavares1, Telma Leda G. De Lemos1, Ângela Martha C. Arriaga1*, Maria Valdeline S. Teixeira1, Gilvandete Maria P. Santiago1,2 And Daniele A. Ferreira1
1Programa de Pós-Graduação em Quimica, Departamento de Química Orgânica e Inorgânica, Universidade Federal do Ceará, 60451-970 Fortaleza – CE, Brazil.
2Departamento de Farmácia, Universidade Federal do Ceará, 60430-370 Fortaleza – CE, Brazil.
DOI : http://dx.doi.org/10.13005/ojc/300211
Article Received on :
Article Accepted on :
Article Published : 07 Jun 2014
The reduction of nitroaromatic compounds is an important transformation that be used in the synthesis of amines which are interesting intermediates of pharmaceuticals and other derivatives. Linseed, Linum usitatissimum L., were used to catalyze biotransformations of nitrobenzene, ortho-nitroacetophenone, meta- nitroacetophenone and para-nitroacetophenone. Ortho-aminoacetophenone, and para- aminoacetophenone were obtained with excellent chemoselectivity (92.1 to ≥99%).
KEYWORDS:Linum usitatissimum L; Linseed; Bioreduction; Nitroaromatic Compounds; Chemoselectivity.
Download this article as:| Copy the following to cite this article: Tavares L. C, T. L. G.De Lemos,Arriaga A. M. C, Teixeira M. V. S,Santiago G. M. P, D. A Ferreira. Linseed (Linum usitatissimum L.) as a biocatalyst for reduction of nitroaromatic compounds. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Tavares L. C, T. L. G.De Lemos,Arriaga A. M. C, Teixeira M. V. S,Santiago G. M. P, D. A Ferreira.. Linseed (Linum usitatissimum L.) as a biocatalyst for reduction of nitroaromatic compounds. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3718 |
INTRODUCTION
Nitroaromatic compounds are released into the biosphere in the form of chemical or biological waste, but others, that are used as synthetic intermediates, pesticides, explosives, antibiotics, etc. 1-2. Although a few nitroaromatic compounds are from natural source, such as chloramphenicol and some plants of the genus Astragalus synthesize nitro glycosides as a defense mechanism3-4. However, the vast majority of nitroaromatic compounds are derived from anthropogenic sources, mainly as a result of industrial processes1. This class, including nitrofurans, nitropyrenos, nitrobenzenes and various others, is the precursor to the corresponding amines by reduction and has been used in various applications such as pharmaceuticals, antimicrobial agents, food additives and materials in various industrial processes, such as production of plastics, explosives, dyes and agricultural products, etc. 1, 5-7. Since the last century, large areas of soil and groundwater have been heavily contaminated by xenobiotics, thus, several articles have specially addressed the effective forms of biodegradation or biotransformation of these serious environmental contaminants 8-10.
Therefore, nitrocompounds have attracted considerable attention because of their potential risk to human health11. Nevertheless, the use of these compounds has never discontinued since continued being used in various therapeutic classes, such as antibacterial, anti-inflammatory, antihypertensive, antineoplastic and used in veterinary medicine12-14.
Enzymatic reduction is essential for nitrocompounds to exert their therapeutic and/or cytotoxic effects and most of these compounds have been subjected to bioreduction by microorganisms such as bacteria and fungi2, 8. The nitroreduction is the initial step in the catabolism of a variety of nitroaromatic compounds1, 15. The nitroreductase comprise a family of evolutionarily conserved proteins that were originally discovered in eubacteria15. These enzymes are able to catalyze the reduction of the nitro group using and flavin mononucleotide (FMN) or flavin adenine dinucleotide (FAD) as a prosthetic group, and nicotinamide adenine dinucleotide (NAD(P)H) as reducing agent16. These proteins have attracted a great interest and environmental importance to human health, decreasing the toxicity of nitrocompounds, as well as, being applied in biotechnology, bioremediation and biocatalysis17-18.
Studies report biotransformation of nitroaromatic compounds using Arracacia xanthorrhiza and Beta vulgaris associated with a microorganism Candida guilliermondii, coconut water which were converted into amines and to the corresponding acetamides. Some plants, as Lens culinaris seeds, are also reported as a biocatalyst for reducing nitro groups19- 21.
Linum usitatissimum L., flaxseed or linseed has a high content of polyunsaturated omega-3 fatty acids, such as α-linolenic acid. It is considered good source of protein, dietary fiber, as well as, of phenolic acids, flavonoids, vitamins and minerals with antioxidant, antimicrobial and anticancer activities; being used as a functional food for prevention of cardiovascular diseases22.
The reduction of nitrocompounds is important in the production of amines, which can be used as pharmaceutical intermediates21. In recent years, plant parts and microorganisms have been related as a green alternative to reduce nitroaromatic compounds9,17,21,23. Therefore, in the order to find new sources of nitroreductases, we investigated the use the whole seeds of Linum usitatissimum L. for the bioreduction of nitroaromatic substrates (Scheme 1). Then, this work can provide a promising agent for the bioreduction of the nitro group.
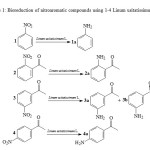 |
Scheme 1: Bioreduction of nitroaromatic compounds using 1-4 Linum usitatissimum L. seeds. Click here to View Scheme |
MATERIAL AND METHODS
Plant material
Commercial seeds of Linum usitatissimum L. were purchased in a local market. Botanical identification data were provided by the Celeiro Alimentos e Produtos Naturais Ltda. (celeiro@celeiroalimentos.com.br).
General Experimental Procedure
The nitroaromatic compounds (1-4) were obtained from Sigma-Aldrich provider. Whole seeds of Linum usitatissimum L. were washed with 5% sodium hypochlorite and rinsed with sterile distilled water. Each nitroaromatic compounds substrate, 1-4, (50 mg), were added to a suspension of 20 g of L. usitatissimum L. seeds in a 40 ml of a buffered solution (Na2HPO4/KH2PO4), pH 6.0, and incubated at 25°C, in an orbital shaker (175 rpm) for 72 hours. All biotransformation reactions were performed using the methodology proposed by Machado et. al. modified24. The reactions were performed in triplicate. The course of all reactions was monitored by TLC (Merck, Silica gel 60 F254) and the substances revealed by spraying with vanillin solution. After completion of the reaction, the each suspension was filtered, washed with water and the aqueous solutions were extracted with CH2Cl2 (3 x 50 mL). The organic phases were dried with Na2SO4 and removed in a rotator evaporator. The reaction products were purified by column chromatography on silica gel 60 VETEC, with a binary mixture of hexane/ethyl acetate (8:2, v/v) as eluente.
All reaction products were analyzed by High Performance Liquid Chromatography (HPLC) of Shimadzu, L201147 pump equipped with a chiral columns OB-H and AS-H, mobile phase binary mixture of n-hexane/isopropyl alcohol (90:10 to 98:2) ranging the composition of the same according to the needs of the sample, and Shimadzu UV-Vis detector SPD-M20A. The mass spectra were obtained using a Shimadzu gas chromatograph GC-2010 plus model coupled to a mass spectrometer of Shimadzu GCMS-QP model SE 2010, using a column with a stationary phase of 95% dimethylpolysiloxane/5% diphenylpolysiloxane with a length of 30 m, internal diameter 0.25 mm, outer diameter of 0.39 mm and 0.25 µm of film thickness and an auto-injector of Shimadzu AOC-20i model. The mobile phase was helium. The values of conversion and enantiomeric excess of biocatalytic reactions were calculated by calibration curve of the starting substrate and the final product prepared in HPLC and verified with mass spectra.
The optical rotation of the product (S)-1-(3-aminophenyl)ethanol (3) was measured in digital Perkin-Elmer 241 and compared with the literature data25.
RESULTS AND DISCUSSION
The biocatalytic reductions of nitroaromatic compounds, nitrobenzene (1), o-nitroacetophenone (2), m-nitroacetophenone (3) and p-nitroacetophenone (4) were performed using whole seeds of Linum usitatissimum L. (Table 1). The compounds were converted into the corresponding amines in good concentrations, with exception of substrate 3. The reactions of bioreductions of the compounds 1, 2 and 4 shown high values of conversion of a nitro group to amino group (92.1%, ≥99% and 98.1%, respectively), then the steric influence was not observed due the presence of the substituents on aromatic ring as observed when the Lens culinaris was used as biocatalyst21. These ones for the compounds 2 and 4 shown high chemoselectivity, the carbonyls were unaffected and only the aromatic amine was produced. Chemoselectivity was also observed in some reactions with vegetables20, 26 but only the carbonyl was reduced; also the literature reports the bioreduction of nitroaromatic compounds21 but with the rather modest yields. Finally, the reaction of substrate 3, with the Linum usitatissimum L. seeds, gave rise to 3-amino-acetophenone (3a) and (S)-1-(3-aminophenyl)ethanol (3b) as products, with values of conversion of 21.6% e 40.5%, respectively. The presence of a carbonyl group, above all when it is in para or ortho position, that decreases the electron deficiency in nitroaromatic ring, appears favor the reduction of the nitro group19. In addition, we observed low enantioselectivity (8.7%) of the product alcohol (3b) with the S configuration, which is in agreement with Prelog rule24, 26.
Linum usitatissimum L. seeds showed a suitable biocatalyst for the preparation of aromatic amines of industrial and pharmaceutical interest from nitroaromatic compounds, with an excellent chemoselectivity (92.1 to ≥99%). When a carbonyl is located in meta position relative to the nitro group, it was also reduced with a medium conversion (21.6 and 40.5%) and low enantioselectivity (8.7%). The results obtained suggest that L. usitatissimum L. has a nitroreductase system that is responsible for the reductions observed, and it can be considered a potential biocatalyst for applications in academic organic synthesis, as well as, in the chemical and pharmaceutical industries.
Table 1: Results of reductions of nitroaromatic compounds (1-4) using the seeds of Linum usitatissimum L.
|
Substrates |
Bioconversion amine (%) |
Bioconversion amine and alcohol (%) |
ee (%) |
|
1 |
92.1 |
– |
– |
|
2 |
≥99 |
– |
– |
|
3 |
21.6 |
40.5 |
8.7 |
|
4 |
98.1 |
– |
– |
Aminobenzene (1)
GC-MS m/z (%) 93 (M+, C6H7N; 100), 93 (100), 66 (50); 39 (15) HPLC: Chiralcel OB-H, 30ºC, n-hexane/isopropyl alcohol (95:5), 0.8 mL/min, tR (min): 12.2.
2-aminoacetophenone (2)
GC-MS m/z (%) 135 (M+, C8H9NO; 80), 120 (100), 92 (50), 65 (30), 39 (10); HPLC: Chiralcel AS-H, 35ºC, n-hexane/isopropyl alcohol (80:20), 1 mL/min, tR (min): 9.4.
3-aminoacetophenone (3a)
GC-MS m/z (%) 135 (M+, C8H9NO; 90), 120 (90), 92 (100), 65 (40), 39 (15); HPLC: Chiralcel OB-H, 30ºC, n-hexane/isopropyl alcohol (95:5), 0.5 mL/min, tR (min): 17.3.
(S)-1-(3-aminophenyl)ethanol (3b)
-5.8° (c. 0.01, CHCl3); GC-MS m/z (%) 137 (M+, C8H11NO; 25), 119 (100), 94 (50), 39 (10); HPLC: Chiralcel AS-H, 30ºC, n-hexane/isopropyl alcohol (97:3), 1 mL/min, tR (min): 21.4 (S), 23.6 (R).
4-aminoacetophenone (3a)
GC-MS m/z (%) 135 (M+, C8H9NO; 50), 120 (100), 92 (45), 65 (30), 39 (10); HPLC: Chiralcel AS-H, 30ºC, n-hexane/isopropyl alcohol (85:15), 1 mL/min, tR (min): 14.0.
ACKNOWLEDMENTS
The authors thank to the Brazilian agencies CAPES and CNPq for fellowships and financial support.
REFERENCES
- Spain J. C., Annu. Rev. Microbiol., 49, 523-55 (1995).
- Race P. R., Lovering A. L., Green R. M., Ossor A., White S. A., Searle P. F., Wrighton C. J., Hyde E. I., J. Biol. Chem., 280, 13256–64 (2005).
- Aouiche A., Sabaou N., Meklata A., Zitouni A., Bijani C., Mathieu F., Lebrihi A., World J. Microb. Biot., 28, 943-51 (2012).
- Aanderson R. C., Rasmussen M. A., Allison M. J., Appl. Environ. Microbiol., 59, 3056-61 (1993).
- Comasseto J. V., Assis L. F., Andrade L. H., Schoenlein-Crusius I. H., Porto A. L. M., J. Mol. Catal. B: Enzym., 39, 24-30 (2006).
- Traversi D., Degan R., De Marcos R. Gillig G., Pignata C., Villani S., Bono R. Environ. Int., 35, 905-10 (2009).
- Mir N. A., Haque M. M., Khan A., Muneer M., Boxall C., Sci. World J., 2012, 1-8 (2012).
- Kou-san J., Parales R. E., Microbiol. Mol. Biol. Rev., 74, 250-72 (2010).
- Esteve-Núñez A., Caballero A., Ramos J. L., Microbiol. Mol. Biol. Rev., 65, 335-52 (2001).
- Nyanhongo G. S., Schroeder M., Steiner W., Gübitz G. M., Biocatal. Biotransform., 23, 53-69 (2005).
- Boelsterli U. A., Ho H. K., Zhou S., Leow K. Y., Curr. Drug Metab., 7, 715-27 (2006).
- Paula F. R., Serrano S. H. P., Tavares L. C. Quim. Nova, 32, 1013-20 (2009).
- Hoorrocks S. M., Jung Y. S., Huwe J. K., Harvey R. B., Ricke S. C., Carstens G. E., Callaway T. R., Anderson R. C., Ramlachan N., Nisbet D. J., J. Food Sci., 72, M50-55 (2007).
- Buschini A., Ferrarini L., Franzoni S., Galati S., Lazzaretti M., Mussi F., Alburquerque C. N., Zuchi T. M. A. D., Poli P., J. Parasitol. Res., 2009, 1-11 (2009).
- Roldan M. D., Perez-Reinado E., Castillo F., Moreno-Vivian C. FEMS Microbiol. Rev., 32, 474-500 (2008).
- Oliveira I. M. and Bonatto D., Henriques J. A. P., Curr. Res., Technol. Educ. Top. Appl. Microbiol. Microbial Biotechnol., 2, 1008-19 (2010).
- Hannink N., Rosser S. J., French C. E., Basran A., Murray J. A., Nicklin S., Bruce N. C., Nature Biotechnol., 19, 1168-72 (2001).
- Kadiyala V., Nadeau L. J., Spain J. C., Appl. Environ. Microbiol., 69, 6520-26 (2003).
- Pacheco A. O., Kagohara E., Andrade H. L., Comasseto J. V., Crusius I. H. –S., Paula C. R., Porto A. L.M., Enzyme Microbial Technol., 42, 65-9 (2007).
- Suárez-Franco G., Hernández-Quiroz T., Navarro-Ocaña A., Oliart-Ros R. M., Valerio-Alfaro G., Biotechnol. Bioproc. Eng., 15, 441-5 (2010).
- Ferreira D. A., Silva R. C., Assunção J. C. C., Mattos M. C., Lemos T. L. G., Monte F. J. Q., Biotechnol. Bioproc. Eng., 17, 407-12 (2012).
- Couto A. N., Wichmann F. M. A., Alim. Nutr., 22, 601-8 (2011).
- Bizerra A. M. C., Gonzalo G., Lavandera I., Gotor-Fernandez V., de Mattos, M. C., de Oliveira, M. C. F., Lemos T. L. G., Gotor V., Tetrahedron. Assym. 21, 566-70 2010).
- Machado L. L., Souza J. S. N., Matos M. C., Sakata S., Cordell G. A., Lemos T. L. G., Phytochem., 67, 1637- 43 (2006).
- Yu S., Quan-yuan H., Shao-rong Y., Xian-ming H., Wuhan Univ. J. Nat. Sci., 7, 113-6 (2002).
- Assunção J. C. C., Machado L. L., Lemos T. L. G., Cordell G. A., Monte F. J. Q., J. Mol. Catal. B: Enzym., 52-53, 194-8 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









