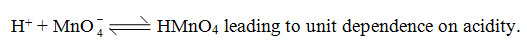Kinetics and Mechanism of Oxidation of Phenyl Acetic Acid and Dl-Mandelic Acid by Permanganate in Acid Medium
K.Venkata Ratnam1*, B.Syama Sundar2, P.S.Radhakrishna murti3
1. Department of chemistry, Gitam University, Bengaluru campus, Doddaballpur, India 561203
2. Vice-Chancellor, Yogi Vemana University, Kadapa, India-516003
3. Department of chemistry, T.J.P.S College (PG Courses), Guntur, India-522006
DOI : http://dx.doi.org/10.13005/ojc/300238
Article Received on :
Article Accepted on :
Article Published : 27 Jun 2014
Kinetics of oxidation of phenyl acetic acid and DL- Mandelic acid by potassium permanganate in aqueous acetic acid and perchloric acid mixture reveals that the kinetic orders are first order in oxidant, first order in H+ and zero order in substrate for phenyl acetic acid. DL-Mandelic acid exhibits first order in oxidant and zero order in substrate. The results are rationalised by a mechanism involving intermediate formation of mandelic acid in case of Phenyl acetic acid and ester formation with Mn (VII) in case of DL-Mandelic acid. The following order of reactivity is observed: DL-Mandelic acid > Phenyl acetic acid. The high reactivity of DL-Mandelic acid over phenyl acetic acid may be due to different mechanisms operating with the two substrates and benzaldehyde is the final product in both the cases.
KEYWORDS:Kinetics; Oxidation; Phenyl acetic acid; DL-mandelic acid; Permanganate; Acid medium
Download this article as:| Copy the following to cite this article: Ratnam K. V, Sundar B. S, murti P. S. R. K. Kinetics and Mechanism of Oxidation of Phenyl Acetic Acid and Dl-Mandelic Acid by Permanganate in Acid Medium. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Ratnam K. V, Sundar B. S, murti P. S. R. K. Kinetics and Mechanism of Oxidation of Phenyl Acetic Acid and Dl-Mandelic Acid by Permanganate in Acid Medium. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3983 |
INTRODUCTION
A survey of literature shows that reports on kinetics of oxidation of Phenyl acetic acid and DL-Mandelic acid by various oxidants are well documented1-22. But it clearly indicates that there is no comparative studies on oxidation of Phenyl acetic acid and DL- Mandelic acid under similar conditions by Mn(VII). Hence to establish the differential reactivity of the above substrates oxidation by Mn(VII) in acidic medium has been investigated. This was also useful in understanding the differential mechanistic pathways in these oxidations, nature of valence states and role of solvent.
MATERIAL AND METHODS
Potassium permanganate – BDH (AR) was prepared in doubly distilled water and used as stock solution.
Phenyl acetic acid – BDH (AR) was used without further purification.
DL-mandelic acid – BDH (AR) was used without further purification.
All other chemicals, acids and solvents used in these investigations were of analytical reagent grade.
Kinetic method
The method employed by Tompkins22 was used: 5.0 ml aliquots of the reaction solution to 5% KI solution acidified with 2N H2SO4 and the liberated iodine was titrated with standard thiosulpahte solution. To reduce aerial oxidation of the acidified iodide solution, the acid concentration was kept low. When possible, the aliquot was titrated immediately after the withdrawl. In fast runs they were titrated in random order. In all the cases the titrations were completed within 10 minutes of the liberation of iodine. The low concentration of the thiosulphate employed necessitated its standardization frequently with standard dichromate.
Product analysis
Both substrates like Phenyl acetic acid and DL-Mandelic acid are oxidised to give benzaldehyde as the major product. This was identified by 2,4-DNP test and its melting point was found to be 2340C which matches with known value.
RESULTS AND DISCUSSION
Kinetics and mechanism of oxidation of phenyl acetic acid and DL mandelic acid by Mn(VII) in aqueous acetic acid medium in the presence of perchloric acid have been investigated. The kinetics results are given below.
Phenyl acetic acid
Effect of varying concentration of oxidant on reaction rate
The reactions are found to be first order with respect to the oxidant. The first order nature with respect to oxidant is confirmed from the constancy in the rate constants at different initial concentrations of oxidant. Moreover a plot of log(a-x) vs time is linear confirming the first order dependence on oxidant (Table-I).
Effect of varying concentration of substrate on reaction rate
The dependence on the substrate has been found to be zero order as the first order rate constants do not change with increase in concentration of substrate (Table-I).
 |
Table1: Effect of variation of various constituents on reaction rate of Phenyl acetic acid |
Effect of varying concentrations of acid on reaction rate
The kinetic data relating to changes in rate constants with change in acidity is recorded in Table-I. A plot of log k vs log H+ is linear with unit slope. It appears that the active species is permanganic acid as per the equilibrium.
Formula
Effect of change in dielectric constant of the medium
The effect of change in solvent composition of acetic acid on reaction rate has been investigated. The rate constants are presented in Table-I. The reaction rate increases with decrease in dielectric constant. A plot of log k vs D-1/2D+123,24 is linear. This shows that these are essentially dipolar – dipolar reactions with the proviso that specific solvent influences like micro dielectric constant at the reaction site, molecular cohesion and other factors like solute – solvent interactions are operating in these reactions finally leading to increase in rate with decrease in dielectric constant.
Effect of addition of salts
The effect of addition of salts like Mn++ and NaF has been investigated (Table-I). Retardation has been observed with both the salts. The reduction observed with Mn++ is due to the reduction of effective concentration of MnO as per the equation
4 Mn(II) + Mn(VII) → 3 Mn(IV) + Mn(III).
This indicates that the main oxidant species is Mn(VII). The retardation observed with F– normally is attributed to the presence of competing intermediate species like Mn(IV) or Mn(III). But the higher retardation with Mn++ shows preferentially the main species is Mn(VII) with the other oxidation states like Mn(IV) or Mn(III) being comparatively less in concentration.
DL- Mandelic acid:
Effect of varying concentration of oxidant on reaction rate
The reactions are found to be first order with respect to the oxidant. The first order nature with respect to oxidant is confirmed from the constancy in the rate constants at different initial concentrations of oxidant. Moreover a plot of log (a-x) vs time is linear confirming the first order dependence on oxidant (Table-II).
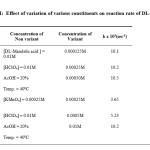 |
Table2: Effect of variation of various constituents on reaction rate of DL-Mandelic acid |
Effect of varying concentration of substrate on reaction rate
The dependence on the substrate appears to be zero order at all concentrations of substrate as forty fold increase in concentration of the substrate results in 3-fold increase only in reaction rate. This points out that the reaction is apparently of zero order in substrate as found with phenyl acetic acid (Table-II).
Effect of varying concentrations of acid on reaction rate
The effect of variation of H+ in this oxidation could not be studied due to the enormous fastness of reaction with increasing acidity.
Nature of oxidation states of manganese
Manganese exhibits a variable oxidation number. Oxidation states of manganese i.e. +2, +4 and +7, are stable over wide range of acidity. In basic solution Mn(VI) and Mn(IV) are the main species of manganese.
The redox potentials are given below to give a comparative account of valency changes of manganese. However, the overall potential of Mn(VII) to Mn(II) change is 1.51V.
E0, volts
Mn(VII) – Mn(VI) e + MnO MnO → + 0.558 + 0.002
Mn(VI) – Mn(V) e + MnO MnO → + 0.285 + 0.001
Mn(III) – Mn(II) e + Mn Mn → + 1.51
In the present investigation it appears that the two electron change in the rate determining step is important followed by fast changes of Mn(V) leading to finally Mn(II) in acid medium.
Mechanistic pathway of oxidation of Phenyl acetic acid:
The kinetic orders observed are first order in oxidant, first order
in H+ and zero order in substrate. The initial reaction appears to be intermediate formation of mandelic acid which decomposes to give benzaldehyde in fast step.
Scheme
 |
Scheme 1 Click here to View Scheme |
The final product is benzaldehyde
The sequence given above indicates that the rate determining step involves a two electron loss leading to a carbonium ion which rapidly reacts with water to give mandelic acid initially. Mandelic acid is rapidly oxidised to give benzaldehyde which is the final product.
Mechanistic pathway of oxidation of DL-Mandelic acid
In the present investigation mandelic acid undergoes oxidative decarboxylation to give benzaldehyde. In acid medium permanganate functions as a two electron oxidant. Hence the ionic pathway through ester formation is preferred. In this connection a comment on the oxidation of mandelic acid by acid permanganate at pH 4.3 by earlier workers25 is necessary. At pH 4.3 the authors postulated a mechanism involving one electron loss leading to radical formation. It is suggested that acid permanganate essentially reacts through a two electron transfer leading to an ionic pathway. It is only in alkaline medium a one electron loss is postulated in permanganate oxidations. It is difficult to arrive at any of the three types of decarboxylation with ionic path way on the basis of kinetic data alone. In the present investigation the pathway is as follows.
Scheme2
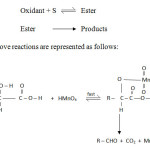 |
Scheme2 Click here to View Scheme |
Comparison of oxidation of phenyl acetic acid and Mandelic acid:
Mandelic acid reacts much faster than phenyl acetic acid. The presence of hydroxy group helps in simultaneous oxidation and decarboxylation leading to faster rate. Phenyl acetic acid undergoes initial slow oxidation to mandelic acid which later is oxidized rapidly to benzaldehyde. The corresponding rate constants are 1.08 x 103 sec-1 and 10.22 x 103 sec-1 for phenyl acetic acid and mandelic acid respectively. The high reactivity of mandelic acid over phenyl acetic acid may be due to different mechanisms operating with the two substrates. The former may be passing through a rate determining O – H bond cleavage through ester formation and the latter by C – H bond fission.
REFERENCES
- Trahnovsky,W.S.; Cramer, J.; and Brixius , D.W. J.Am.Chem.Soc. 1974 , 96, 1078.
- Ramanandasarma, Y.; Rajanna,K.C.; Saiprakash, P.K. Indian J.Chem. 1980, 19A, 872.
- Vausdevan,R.; Subramanian, P.S.; Mathai, I.M. J.Indian.Chem.Soc. 1984, 61,395.
- .Pati,S.C.; Pathy, H.P.; Dev, B.R. Proc.Indian. Natn.Acad. 1985, 51,853.
- Venkata Subramanian, N.; Sundaram,S.; Curr. Sci. 1964, 33, 646.
- Venkata Subramanian, N.; Sundaram,S.; Curr. Sci.1965, 34, 662.
- Banerji, K.K.; Indian. J.Chem. 1977, 15A, 675.
- Banerji, K.K.; Indian. J. Chem. 1979, 17A, 904.
- Malkani, A.K.; Suresh, K.S.; Bakore,G.V. J.Indian.Chem.Soc.1978, LV, 215 – 219.
- Venakat Subramanian, N.; Sundaram, S.; Swaminathan, Indian.J.Chem.1975, 13, 1163.
- Barker, I.R.L.; Aukett, P.J. J.Chem.Soc., Perk-II, 1973, 965.
- Natarajan, R.; Venakata Subramanian, N.; Indian.J.Chem. 1975, 13, 261.
- Kuselan, P.; Venkata Subramanian,N. Indian. J.Chem. 1983, 22A, 292.
- Radhakrishnamurti, P.S.; Rath, N.K.; Panda R.K. Indian.J.Chem. 1988, 27A, 407.
- Brahmaiah; Manikyamba, P.; Indian.J.Chem. 1995, 34(A),900.
- Chandraiah, U.; Murthy, C.P.; Sushma Kandlikar, Indian.J.Chem.1989, 28A, 162.
- VeeraSomaiah, P.; BalReddy, K.; Navaneetha Rao,T. Indian.J.Chem. 1988, 27A, 876.
- Balji Kawle; Thirupathi Rao, M.; Adinarayana,M. Inidan.J.Chem. 1994, 33A, 1021.
- Issifou. Kouadio; Louis J.Kirschembaum; Raj.N.Mehrotra; Yunfusun. 1990, J.Chem.Soc., Perkin Trans2, 2123.
- Donald G.Lee ; Taochen. J.Org.Chem., 1991, 56, 5341.
- Sanjeeva Reddy, Ch.; Vijaya Lakshmi,T. Indian.J.Chem. 1998, 37A, 91.
- Tompkins, F.C.; Trans. Faraday Soc. 1942,38, 131.
- Amis, E.S.; J. Chem. Ed. 1953, 30, 351.
- Amis, E.S.; Anal. Chem. 1955, 27, 1672.
- Bakore, G.V.; Rama Shankar;Urmila Goyal.; Indian J.Chem. 1963, 1, 331.

This work is licensed under a Creative Commons Attribution 4.0 International License.