Unfolding-Induced in Haemoglobin by Exposure to Electromagnetic Fields: a Ftir Spectroscopy Study.
Emanuele Calabrò1,*, Salvatore Magazù1
1Department of Physics and of Earth Science, University of Messina, Italy
DOI : http://dx.doi.org/10.13005/ojc/300104
Article Received on : January 01, 2014
Article Accepted on : February 06, 2014
Article Published : 13 Mar 2014
The effects of extremely low frequency electromagnetic field on the secondary structure of hemoglobin were investigated by means of Fourier Transform Infrared Spectroscopy. A decrease in intensity of the α-helix component in the amide I and amide II regions was observed after exposure of 4 h to a 50 Hz electromagnetic field at 1 mT. In addition, Fourier self deconvolution analysis was carried out on exposed and not-exposed spectra. A relative increase of the β-sheet feature in the amide I region was evidenced, showing that an unfolding process of the protein occurred after exposure to extremely low frequency electromagnetic field, suggesting the hypothesis of the formation of aggregates.
KEYWORDS:hypothesis; Several; epidemiological; studies have reported possible evidence.
Download this article as:| Copy the following to cite this article: Calabro E, Magazu S . Unfolding-Induced in Haemoglobin by Exposure to Electromagnetic Fields: a Ftir Spectroscopy Study. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Calabro E, Magazu S . Unfolding-Induced in Haemoglobin by Exposure to Electromagnetic Fields: a Ftir Spectroscopy Study. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2422 |
Introduction
Several epidemiological studies have reported possible effects on human health from exposure to extremely low frequency electromagnetic field (ELF-EMF). In response to public concern over health effects of EMF exposure, in 1996, the International EMF Project was established by WHO and the Radiation and Environmental Health Unit, which coordinated studies on EMF relative to the Environmental Health Criteria (EHC). In particular, the International Agency for Research on Cancer (IARC) formally evaluated the evidence for carcinogenesis from exposure to static and ELF-EMFs, concluding that ELF-EMFs are possibly carcinogenic to humans [1]. Milham and Ossiander have suggested that the appearance of the peak incidence at around age 3 in childhood acute lymphocytic leukemia is linked to electrification [2]. Further significant concern has been raised about the capacity of EMFs to cause DNA damage and chromosomes aberrations [3-6]. National radiation advisory authorities recommends measurements to minimize exposure to their citizens, as the exposure limits to electromagnetic fields recommended by the International Commission on Non-Ionizing Radiation Protection (I.C.N.I.R.P.) [7].
However, we focused our attention on proteins. Indeed, proteins are fundamentals in organic metabolism of livings. In the cells each protein must fold into the specific conformational state in a complex and highly crowded environment, and the folding process is aided by a range of auxiliary proteins [8, 9].Otherwise it was largely demonstrated that several type of environmental stress agents can alter the secondary structure of proteins. Recently it was proved that also ELF-EMFs and MWs can alter the secondary structure of proteins [10-16]. Haemoglobin is a heme-protein whose physiological importance is mainly related to its ability to bind molecular oxygen. The oxygen carried by heme-proteins is bound directly to the ferrous iron atom of the heme prosthetic group. The heme portion of hemoglobin is extremely important because it aids in oxygen binding. Haemoglobin is a tetrameric heme-protein found in erythrocytes where it is responsible for binding oxygen transporting the bound oxygen throughout the body to be used in aerobic metabolic functions. The aim of this work was to investigate the alteration produced by exposure to EMFs on the secondary structure of this heme-protein that perform an important role in metabolism processes of organic systems, focusing the attention on aggregation mechanisms. Fourier Transform Infrared (FTIR) Spectroscopy was used to investigate the effects of EMFs in the secondary structure of this protein in aqueous solution, in particular Amide I and II modes in the range 1700-1500 cm-1. Indeed, it may be considered the most versatile spectroscopic technique for analyzing the secondary structure of a protein in diverse physiochemical environments.
Materials and Methods
Haemoglobin samples were obtained as previously reported [10]. The exposure system for haemoglobin consisted of a couple of Helmholtz coils, with pole pieces of round parallel polar faces, to produce a uniform magnetic field at the center of the coils distance. A magnetic flux density of 1 mT between the polar faces of the coils was generated by means of an AC voltage regulating up to 230 V. Samples were placed at the center of a uniform field area between the coils and the magnetic field was continuously monitored by a magnetic field probe GM07 of HIRST Magnetic Instruments Ltd, UK. Analogue unexposed samples at the same room temperature were used as the control. FTIR spectra were recorded by a spectrometer, Vertex 80v, from Bruker Optics. The attenuated total reflection (ATR) method was chosen for spectrum collection. For each spectrum, 64 interferograms were collected with a spectral resolution of 4 cm-1 in the range from 4000 to 1200 cm-1, using the techniques accurately described in [11]. Either exposed or control samples were located in the same room at a temperature of 20°C.
Results and discussion
Exposure of Haemoglobin to 50 Hz EMF
Samples of 250 μL of hemoglobin in bidistilled water aqueous solutions were exposed for 4 h to a uniform electromagnetic field of 1 mT at the frequency of 50 Hz at a room temperature of 20 °C. Analogue unexposed samples at the same room temperature were used as the control. Typical spectra from 1800 to 1400 cm-1, obtained after exposure, are showed in Figure 1. The spectra exhibited an intense amide I band centered at about 1654 cm-1, corresponding mainly to an α-helix structure content due to C═O stretching vibration and a N–H bending mode, a low intensity amide II, coupling of the N–H bending and C–N stretching modes.
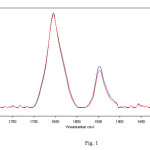 |
Fig. 1 – Representative infrared spectra from 1800 to 1400 cm-1 of hemoglobin in bidistilled water solution after 4 h of exposure to 50 Hz frequency EMF at 1 mT (red lines represent exposed samples spectra). The amide I and II regions are evidenced. The decrease in intensity after exposure was significant in particular in the amide II region. Click here to View Figure |
A significant decrease in intensity of the amide I and II modes was evidenced for exposed sample spectra, that can be due to a decrease of the α-helix component, a loss of α-helical and short-segment connecting α-helix segments in the amide I region. In addition, a perceptible increase of the β-sheet content in the region 1635-1610 cm-1 can be observed in Figure 1, as well. Hence, Fourier self deconvolution (FSD) analysis was used to highlight the alterations in the amide I region. The concept of FSD is based on the assumption which a spectrum of single narrow bands is broadened in the liquid or solid state and cannot be distinguished in the amide envelope. A curve fitting procedure can be applied to estimate quantitatively the area of each component representing a type of secondary structure. After FSD analysis on exposed and not-exposed spectra, vector normalization was used.This analysis revealed the presence of five vibration bands centered at 1654, and around 1635, 1625, 1615 and 1685 cm-1 in the amide I region, as can be observed in Figure 2.
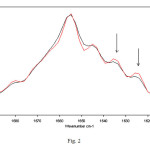 |
Fig. 2 – Representative FSD infrared spectra in the amide I region of hemoglobin in bidistilled water solution after 4 h of exposure to 50 Hz frequency EMF at 1 mT. The relative increases in intensity of β-sheet features (indicated by arrows) after exposure were evidenced by FSD analysis. Red lines represent exposed samples spectra. Click here to View Figure |
The band at 1654 cm-1 is due to α-helix structures, and the other vibrations can be associated with β-sheet structures [17,18]. This analysis relative to hemoglobin in bidistilled water aqueous solution revealed a significant increase in β-sheet bands after 4 h of exposure, indicated with arrows in Figure 2, comparing exposed and unexposed spectra. These features can be attributed to the formation of aggregates [19].
In conclusion, an unfolding process of hemoglobin due to ELF-EMF exposure was enhanced and confirmed by FSD analysis.
Conclusion
The effects of exposure of 4 h to 50 Hz EMF at 1 mT on hemoglobin aqueous solutions were studied by means of FTIR techniques, showing that ELF-EMF can affect infrared vibration bands of hemoglobin. In particular, a loss of α-helical and short-segment connecting α-helix segments was observed in amide I and amide II regions. In addition, the use of FSD analysis evidenced a relative increase in intensity of the β-sheet content with respect to α-helix component in the amide I region, that can be attributed to the formation of aggregates. Previous literature have indicated that the propensity to cause the transition from α-helix to β-sheet structure can be responsible for aggregation leading to the neurotoxicity and neurodegenerative disorders that can be considered as the first step to some pathologies. Hence, further research is needed to highlight bioprotective mechanisms against the effects of ELF-EMF.
References
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Non-ionizing radiation, Part 1: Static and extremely low-frequency (ELF) electric and magnetic fields; Monographs on the Evaluation of Carcinogenic Risks to Humans, 80; IARC: Lyon, 2002.
- S. Milham, E.M. Ossiander, “Historical evidence that residential electrification caused the emergence of the childhood leukemia peak”, Med. Hypotheses Vol. 56 (3), pp. 290–295, 20015
- I. Y. Belyaev, C. B. Koch, O. Terenius, K. Roxstrom- Lindquist, L. O. Malmgren, W. H. Sommer, L. G. Salford and B. R. Persson, “Exposure of Rat Brain to 915 MHz GSM Microwaves Induces Changes in Gene Expression but not Double Stranded DNA Breaks or Effects on Chromatin Conformation,” Bioelectromagnetics, Vol. 27, No. 4, pp. 295-306, 2006.
- E. Calabrò, S. Condello, S. Magazù and R. Ientile “Electromagnetic fields low levels altered the DNA infrared region in RA-differentiated SH-SY5Y neuroblastoma cells”, BioTechnology: An Indian Journal Vol. 6, No.8-9, pp. 267-271, 2012.
- E. Diem, C. Schwarz, F. Adlkofer, O. Jahn and H. Rudiger, “Non-thermal DNA Breakage by Mobile-Phone Radiation (1800MHz) in Human Fibroblasts and in Transformed GFSH-R17 Rat Granulosa Cells in Vitro”, Mutation Research Vol. 583 (2), pp. 178-183, 2005.
- R. R. Tice, G. G. Hook, M. Donner, D. I. McRee and A. W. Guy, “Genotoxicity of Radiofrequency Signals. I. Investigation of DNA Damage and Micronuclei Induction in Cultured Human Blood Cells,” Bioelectromagnetics Vol. 23 (2), pp. 113-126, 2002.
- International Commission on Non-Ionizing Radiation Protection, “Guidelines for Limiting Exposure to Time -Varying Electric, Magnetic, and Electromagnetic Fields (up to 300 GHz),” Health Physics, Vol. 74, No. 7, pp. 494-522, 1998.
- R. J. Ellis and F. U. Hartl, “Principles of Protein Folding in the Cellular Environment,” Current Opinion in Structural Biology, Vol. 9, No. 1, pp. 102-110, 1999.
- M. J. Gething and J. Sambrook, “Protein Folding in the Cell,” Nature, Vol. 355, No. 6355, pp. 33-45, 1992.
- S. Magazù, E. Calabrò and S. Campo, “FTIR Spectroscopy Studies on the Bioprotective Effectiveness of Trehalose on Human Hemoglobin Aqueous Solutions under 50 Hz Electromagnetic Field Exposure,” The Journal of Physical Chemistry B, Vol.114, No. 37, pp. 12144-12149, 2010.
- S. Magazù and E. Calabrò, “Studying the Electromagnetic-Induced Changes of the Secondary Structure of Bovine Serum Albumin and the Bioprotective Effectiveness of Trehalose by FTIR Spectroscopy,” The Journal of Physical Chemistry B, Vol. 115, No.21, pp. 6818–6826, 2011.
- E. Calabrò, S. Magazù, “Inspections of Mobile Phone Microwaves Effects on Proteins Secondary Structure by means of Fourier Transform Infrared Spectroscopy”, Journal of Electromagnetic Analysis & Applications Vol. 2, No.11, pp. 607-617, 2010.
- S. Magazù, E. Calabrò, S. Campo, S. Interdonato, “New Insights into Bioprotective Effectiveness of Disaccharides: a FTIR Study of Human Haemoglobin Aqueous Solutions exposed to Static Magnetic Fields”, Journal of Biological Physics Vol. 38, No.1, pp. 61-74, 2012, DOI: 10.1007/s10867-010-9209-1, published on-line March 9, 2011.
- E. Calabrò and S. Magazù, “Electromagnetic Fields Effects on the Secondary Structure of Lysozyme and Bioprotective Effectiveness of Trehalose”, Advances in Physical Chemistry, Vol. 2012, Article ID 970369, 6 pages, doi:10.1155/2012/970369, 2012.
- E. Calabrò, S. Magazù, “Comparison between conventional convective heating and microwave heating: an FTIR spectroscopy study of the effects of microwave oven cooking of bovine breast meat”, Journal of Electromagnetic Analysis & Applications Vol. 4 (11), pp. 433-439, 2012.
- E. Calabrò, S. Magazù, Unfolding and Aggregation of Myoglobin can be Induced by Three Hours Exposure to Mobile Phone Microwaves: a FTIR spectroscopy study, Spectroscopy Letters: An International Journal for Rapid Communication, Vol.46, Issue 8, pp. 583-589, 2013.
- E. Calabrò, S.Magazù, Non-Thermal Effects of Microwave Oven Heating on Ground Beef Meat Studied in the Mid-Infrared Region by FTIR Spectroscopy, Spectroscopy Letters: An International Journal for Rapid Communication, published online 01 Aug 2013, DOI:10.1080/00387010.2013.828313.
- E. Calabrò, S. Condello, M. Currò, N. Ferlazzo, D. Caccamo, S. Magazù and R. Ientile, Effects of Low Intensity Static Magnetic Field on FTIR spectra and ROS production in SH-SY5Y neuronal-like cells, Bioelectromagnetics 34, pp. 618-629, 2013.
- E. Calabrò, S. Magazù, S. Campo, “Microwave-induced increase of amide I and amide II vibration bands and modulating functions of sodium-chloride, sucrose and trehalose aqueous solutions: The case study of Haemoglobin”, Research Journal of Chemistry and Environment, in press in Vol. 16 (4), 59-67, 2012.
- A. A. Ismail, H. H. Mantch, P.T.T. Wong, “Aggregation of chymotrypsinogen: portrait by infrared spectroscopy”, Biochim. Biophys. Acta Vol.1121, pp. 183–188, 1992.
- T. Lefevre, M. Subirade, “Molecular differences in the formation and structure of fine-stranded and particulate β-lactoglobulin gels”, Biopolymers Vol. 54, pp. 578–586, 2000.
- R. Bauer, R. Carrotta, C. Rischel, L. Ogendal, “Characterization and isolation of intermediates in β-lactoglobulin heat aggregation at high pH”, Biophys. J. Vol. 79, pp. 1030–1038, 2000.

This work is licensed under a Creative Commons Attribution 4.0 International License.









