Recovery of Monomer from Nylon waste powder for its Recycling
Dilip B.Patil1 and Swapnil V.Madhamshettiwar2
1 Department of Chemistry, Institute of Science, Nagpur – 440001, INDIA
2 Department of Chemistry, Sardar Patel Mahavidyalaya, Chandrapur – 442402, INDIA
DOI : http://dx.doi.org/10.13005/ojc/300113
Article Received on :
Article Accepted on :
Article Published : 27 Jan 2014
ABSTRACT
Recovery of monomer hexamethylene diamine(HMD) in the form of dibenzoyl derivative of hexamethylene diamine (DBHMD) from Nylon waste rope powder was carried out by degradation of Nylon waste powder of nylon rope waste.The molecular weight of nylon waste powder was found to be 26582.The minimum amount of nylon waste powder and hydrochloric acid required for maximum recovery of HMD and DBHMD was found to be 5g and 5N,50ml hydrochloric acid respectively. Further it was observed that the maximum time and temperature required for getting maximum yield of DBHMD was 120 minutes and 800C respectively.
KEYWORDS:Nylon waste; Depolymerisation; recovery of monomer; hydrolysis; Hexamethylene diamine (HMD),DBHMD.
Download this article as:| Copy the following to cite this article: Patil D. B, Madhamshettiwar S. V. Recovery of Monomer from Nylon waste powder for its Recycling. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Patil D. B, Madhamshettiwar S. V. Recovery of Monomer from Nylon waste powder for its Recycling. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=1976 |
Introduction
Every year thousand tons of polymers are produced in the country and it has been estimated that over 25% of this produce ends up in the municipal disposal sites every year. Nylon account for less that 5 % of all solid waste, but the value of this scrap represent large percentage of total value of all Solid wastes. There are two ways by which this solid waste is generated.
Large amount of post-used nylon rope and carpet are discarded every year. This waste is disposed of by land filling while small portion is incinerated. This nylon fiber are mainly comprises of Nylon-6 and Nylon 66.
During the production of Nylon fiber1 the waste is generated.
The waste generated mainly of two types2, one is aqueous extractable3 and second is Solid Waste4. Now a day’s all industries are engaged in developing recycling process to overcome financial burden and environmental constraints. Chemical recycling of waste polymer has attracted great attention in recent years as a means of obtaining valuable products.5 There are three ways of chemical recycling of nylon waste powder. The first way is Depolymerisation of polymer into their constituent monomer like Caprolactum, hexamethylene diamine and adipic acid6 or other useful oligomers. The second alternative is the use of Solvent for the extraction of polymer and third way of recycling is to produce thermoplastic mixtures by melt blending the nylon waste.
The Kinetic study of hydrothermal depolymerisation of nylon has been carried out by Tomoko et al7 while the identification of degradation product was done by Hommez and Goethals 8. X-ray diffraction and I.R. spectroscopic investigation of the product of depolymerisation of Nylon was done by Mladeov et al9. In 1931, Chemist at E.I DuPont de Nemours discovered, Nylon 66 which later on became one of the most used fiber material. Nylon 66 is the polyamide formed by the condensation reaction between hexamethylene diamine and adipic acid. Each of the monomer has 6 carbon atoms hence the designation 66. The initial applications of nylon 66 were sewing thread, Parachute fabric and women’s hosiery after World War II, Nylon 66 becomes the most used artificial material with varieties of applications. By 1940s, is was already being used for upholstery and in carpet10
Above mentioned reasons show the considerable importance of recovery of monomer from nylon waste.
Experimental
Material, Chemicals and Reagents
The nylon waste in the form of nylon rope were procured and crushed into fine powder after deep freezing it to increase its brittleness similarly Nylon-66 were specially ordered for this experimental work of A.R. grade was used without further purification, in the powdered form. Hydrochloric acid used for hydrothermal depolymesisation of Nylon waste also of A.R.grade of E.Merck. Benzoyl chloride was used for recovery of hexamethylene diamine in the form of it’s derivative was of A.R.grade of E.Merck. Double distilled and deionized water was used throughout the experiment. It was checked on digital conductometer with conductivity of 1-3μS. A 5N Stock solution of
hydrochloric acid (HCl) was prepared by diluting known volume of pure and concentrated HCl (11.3 N) in 100 ml volumetric flask.
Determination of Molecular weight
The average molecular weight of Nylon -66 waste powder was determined by Ostwald’s viscometer. Solvent which was used to prepare Solution of Nylon-66 was m-Cresol. Various Concentration ranging from 2%, 1%, 0.5%, 0.25% were prepared by dissolving Nylon-66 in m-cresol and flow time was measured. By using the equation [η]=K.Mα molecular weight of Nylon -66 was determined.The values of constant K and α for m-cresol are 2.4 x 10-3 and 0.61 respectively.
Conversion of Nylon waste powder into dibenzoyl derivative hexamethylene diamine (DBHMD)
Conversion of nylon waste into HMD was carried out in a 250 ml three necked round bottom flask fitted with extra long water condenser and Thermometer. The optimum parameters of depolymerization of Nylon – 66, were determined so as to get maximum yield of product. The general procedure adopted were consisting of carrying out thermal hydrolysis of fixed weight of Nylon-66 in three necked round bottom flask by using 5N, Hydrochloric acid on heating mantle for fixed time. After heating time at particular temperature, reaction mixture was cooled and then neutralized with strong solution of sodium hydroxide.
Before neutralization reaction mixture was filtered over vacuum pump to get separate adipic acid and hexamethylene diamine. Hexamethylene diamine in the filtrate which was acidic was neutralized with sodium hydroxide. This neutralization was carried out in such way to make filtrate slightly alkaline. This was checked by litmus paper or pH meter. To this alkaline solution of hexamethylene diamine, AR grade benzoyl chloride was added in small installment with vigorous shaking on shaking machine.
This process converts Hexamethylene diamine into dibenzoyl derivative of hexamethylene diamine (DBHMD) in the form of white crystal. Benzoyl chloride was added till the complete DBHMD is formed. It was then filtered on vacuum pump and then recrystalised with ethanol. The recrystalised DBHMD was dried in oven and then weighted accurately on digital balance. This data was recorded in the form of yield. The recrystalized product , DBHMD were dried in oven and stored in air tight bottle and IR spectra was recorded to confirm nature of functional group present. Similarly Pure hexamethylene diamine was
converted into DBHMD and its IR spectra were also recorded, from two IR Spectra it was concluded both the samples were of DBHMD.
Table 1: Conversion of Nylon waste powder to HMD and DBHMD: Variation of amount of nylon waste powder
Time of reaction : 120 min
Temperature : 800C
Concentration of HCl : 5 N
Volume of HCl : 50 ml
|
Amount of nylon waste powder/g
|
DBHMD/g |
HMD/g |
Amount of Nylon waste reacted/g |
Amount of unreacted nylon waste/g |
|
1 |
0.96 |
0.3437 |
0.6706 |
0.3294 |
|
2 |
1.926 |
0.6896 |
1.3453 |
0.6547 |
|
3 |
3.101 |
1.1102 |
2.1661 |
0.8339 |
|
4 |
2.919 |
1.0451 |
2.0390 |
1.9610 |
|
5 |
2.815 |
1.0078 |
1.9663 |
3.0337 |
|
6 |
2.301 |
0.8238 |
1.6073 |
4.3927 |
Table 2: Conversion of Nylon waste powder to HMD and DBHMD: Variation of time of hydrolysis of nylon waste powder
Amount of Nylon Sample : 3 g
Temperature : 800C
Concentration of HCl : 5 N
Volume of HCl : 50 ml
|
Time/min. |
DBHMD/g |
HMD/g |
Amount of unreacted nylon waste/g |
|
30 |
0.589 |
0.2109 |
2.5886 |
|
60 |
1.562 |
0.5592 |
1.9089 |
|
90 |
1.901 |
0.6806 |
1.6721 |
|
120 |
3.101 |
1.1102 |
0.8339 |
|
150 |
2.802 |
1.0032 |
1.0428 |
Table 3: Conversion of Nylon waste powder to HMD and DBHMD: Variation of temperature of hydrolysis of nylon waste powder
Amount of Nylon Sample : 3 g
Heating time : 120 min
Concentration of HCl : 5 N
Volume of HCl : 50 ml
|
Temperature/0C |
DBHMD/g |
HMD/g |
Amount of unreacted nylon waste/g |
Amount of Reacted nylon waste /g |
|
40 |
2.771 |
0.9921 |
1.0644 |
1.9356 |
|
60 |
3.016 |
1.0798 |
0.8933 |
2.1067 |
|
80 |
3.101 |
1.1102 |
0.8339 |
2.1661 |
|
100 |
3.029 |
1.0845 |
0.8842 |
2.1158 |
Table 4: Conversion of Nylon waste powder to HMD and DBHMD: Variation of Volume of HCl for hydrolysis of nylon waste powder
Amount of Nylon Sample : 3 g
Temperature : 800C
Heating time : 120 min
Normality of HCl : 5 N
|
Volume of HCl/ml |
DBHMD/g |
HMD/g |
Amount of reacted nylon waste /g |
Amount of unreacted nylon waste/g |
|
20 |
0.856 |
0.3065 |
0.5979 |
2.4021 |
|
40 |
1.321 |
0.4730 |
0.9227 |
2.0773 |
|
50 |
3.101 |
1.1102 |
2.1661 |
0.8339 |
|
60 |
3.075 |
1.1009 |
2.1479 |
0.8521 |
|
80 |
3.018 |
1.0805 |
2.1081 |
0.8919 |
|
100 |
3.02 |
1.0812 |
2.1095 |
0.8905 |
Table 5: Conversion of Nylon waste powder to HMD and DBHMD: Molecular weight determination of Nylon waste
|
Concentration % |
Flow time of Solution (t)/ sec. |
Flow time of Solvent (to)/Sec. |
Relative Viscosity |
Specific Viscosity |
Reduced Viscosity |
|
1 |
426 |
170 |
2.5058 |
1.5058 |
1.5058 |
|
0.50 |
269 |
1.5823 |
0.5823 |
1.1646 |
|
|
0.25 |
203 |
1.1941 |
0.1941 |
0.7764 |
|
|
0.125 |
178 |
1.0471 |
0.0471 |
0.3768 |
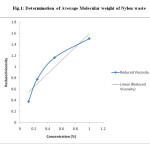 |
Fig.1: Determination of Average Molecular weight of Nylon waste Click here to View figure |
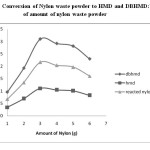 |
Fig.2: Conversion of Nylon waste powder to HMD and DBHMD:Variation of amount of nylon waste powder Click here to View figure |
 |
Fig.3: Conversion of Nylon waste powder to HMD and DBHMD: Variation of time of hydrolysis of nylon waste powder Click here to View figure |
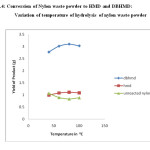 |
Fig.4: Conversion of Nylon waste powder to HMD and DBHMD: Variation of temperature of hydrolysis of nylon waste powder Click here to View figure |
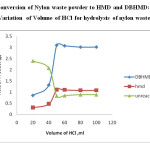 |
Fig 5: Conversion of Nylon waste powder to HMD and DBHMD: Variation of Volume of HCl for hydrolysis of nylon waste powder Click here to View figure |
 |
Fig 6: FTIR of DBHMD Click here to View figure |
Result and Discussion
The average molecular weight of Nylon -66 waste powder was determined by Ostwald’s viscometer. Solvent which was used to prepare Solution of Nylon-66 was m-Cresol. By using the equation [η] =K.Mα molecular weight of Nylon -66 was determined. The values of constant K and α for m-cresol are 2.4 x 10-3 and 0.61 respectively.
The average molecular weight of used nylon waste powder is 26582. [Ref.Fig 1, Table 5]
The result obtained in table 1 and Figure 2 shows that the maximum conversion of nylon waste powder to HMD occurred at 3.0g Nylon powder. The yield obtained is 73%.Further increase in amount of nylon waste, the % conversion of nylon waste powder to HMD decreases and found to be 65% at 4.0g .Hence the maximum conversion obtained at 3.0g of nylon waste powder.
The optimum amount of hydrochloric acid has been found to be 50 ml having concentration of 5N.The variation is reported in Table 4 and figure 5.
The increase in time of hydrothermal depolymerisation increases the conversion of nylon waste into HMD.The maximum conversion took place at 120 minutes.Further increase in the reflux time found to show negligible effect on the yield.
[Ref.Table 2 and Fig. 3]
It is also observed that increase in temperature increases the conversion of nylon waste into HMD.The optimum temperature for the conversion is found to be 800C.[Ref.Table 3 and Fig.4].The HMD obtained after conversion was treated with benzoyl chloride to get dibenzoyl derivative of HMD.It was identified by recording its FTIR.[Ref.Fig. 6].It is observed that spectra of pure DBHMD is similar to spectra of authentic DBHMD.
Conclusion
The molecular weight of nylon waste powder determined by using solvent m-cresol was found to be 26582. The maximum yield of DBHMD was obtained when 3 g of nylon waste powder undergoes hydrothermal depolymerisation. The optimum reflux time recorded for conversion was 120 minutes. The maximum temperature for the conversion is 800C. The hydrothermal depolymerisation of nylon waste powder with 50 ml, 5Nhydrochloric acid gives maximum yield of DBHMD.
References
- L.A.Dmitrieva, V. (1985). Nylon Fibre.
- R.N.Goel. (1982). Synthetic Fibre.
- E.Morf. (30/82 1980). Chemiefasern/Textilindustrire. 506.
- K.R.Wolff. (30/82 1980). Chemiefasern/Textilindustire. 500.
- R.D.Leaversuch. (41,68,1991). Modern Plastics.
- Dupont. (1962). Patent No. 3,609,465. United state of America
- I.Tomoko, S. (2006). Polymer degradation and stability. In 91 (pp. 1989-95).
- B.Hmmez, E. (1998). Journal of macromolecular science , 1489-1505.
- I.Mladenov, T. (1978). Polymer Society. 20,341,USSR.
- A.M.Katlair and D.P.Fountain,Gorgia Tech.,Synthetic wood from waste fibrous materials,U.S.Patent 5,626,939 (1997).

This work is licensed under a Creative Commons Attribution 4.0 International License.









