Organometallic Derivatives of Ruthenium (II & III) Ligated by Quinazole-2-Thione-4-One
R.N. Pandey* and K.V. Gautam
P.G. Centre of Chemistry (M.U.), College of Commerce, PATNA – 800020 (INDIA)
DOI : http://dx.doi.org/10.13005/ojc/300152
Article Received on :
Article Accepted on :
Article Published : 30 Mar 2014
Mixed ligand phosphine and arsine complexes of ruthenium (II & III) ligated by Quinazole-2-thione-4-on (QTH) of composition [Ru(Pf3)2(QT)2], [RuX(CO)(Ef3)2(QT)] and [RuX2(Ef3)2(QT)] (E = P/As; X = Cl/Br; QT = moobasic bidentate N,S-chelating ligand) are prepared and investigated. The identities of complexes are established by elemental analysis, conductivity measurements, magnetic susceptibility and spectral methods (IR, UV-vis, 1H NMR). An octahedral geometry has been tentatively proposed for all these complexes.
KEYWORDS:Ruthenium (II and III) chelates; Heterocyclic Thioamide; Octahedral.
Download this article as:| Copy the following to cite this article: Pandey* N. R and Gautam V. K. Organometallic Derivatives of Ruthenium (II & III) Ligated by Quinazole-2-Thione-4-One. Orient J Chem 2014;30(1). |
| Copy the following to cite this URL: Pandey* N. R and Gautam V. K. Organometallic Derivatives of Ruthenium (II & III) Ligated by Quinazole-2-Thione-4-One. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2575 |
INTRODUCTION
Quinazole-2-thione-4-one (Str. I) contains amide and thioamide groups having interesting donor sites.1 We have reported some complexes of low-valent2 and high-valent3 metal ions with this ligand in our earlier communication. The present paper reports synthesis, spectral characterization and structural investigation of some ruthenium (II & III) chelates with this ligand.
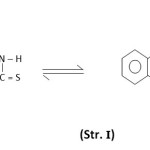 |
Scheme :1 Click here to View Scheme |
EXPERIMENTAL
All chemicals used in this work were either of Anal R grade or chemically pure grade. The Quinazoline-2-thione-4-one (QTH)4 precursor complexes, [RuCl2(Pf3)4]5, [RuHCl(CO)(Pf3)3]6, [RuHCl(CO)(Asf3)3]7, [RuHCl(CO)(Pf3)2(Py)]8, [RuCl3(Pf)3]9, [RuBr3(Pf3)3]10 and [RuCl3(Asf3)3]11 were prepared by reported literature methods.
Preparation of new ruthenium (II) complexes
All new ruthenium (II) complexes of composition [RuCl(CO)(Ef3)2(QT)] (E = As/P) were prepared by the ligand substitution in benzene using precursor complexes and ligand reported in our previous method.12 The other ruthenium (II) complex of composition [Ru(Pf3)2(QT)2] was prepared by direct reaction of [RuCl2(Pf3)4] with ligand (QTH) following our previous method.13
Analysis
[RuCl(CO)(Pf3)2(QT)] (yellow) : Calculated (%) for RuC45H35N2O2P2SCl (865.5) : C = 62.39; H = 4.04; N = 3.23; Cl = 4.10; Ru = 11.66; Found (%) : C = 62.42; H = 4.10; N = 3.33; Cl = 4.21; Ru = 11.55;
[RuCl(CO)(Asf3)2(QT)] (yellow) : Calculated (%) for RuC45H35N2O2As2SCl (953.5) : C = 56.63; H = 3.74; N = 2.99; Cl = 3.72; Ru = 10.59; Found (%) : C = 56.11; H = 3.77; N = 3.01; Cl = 80; Ru = 10.32;
EXPERIMENTAL
All chemicals used in this work were either of Anal R grade or chemically pure grade. The Quinazoline-2-thione-4-one (QTH)4 precursor complexes, [RuCl2(Pf3)4]5, [RuHCl(CO)(Pf3)3]6, [RuHCl(CO)(Asf3)3]7, [RuHCl(CO)(Pf3)2(Py)]8, [RuCl3(Pf)3]9, [RuBr3(Pf3)3]10 and [RuCl3(Asf3)3]11 were prepared by reported literature methods.
Preparation of new ruthenium (II) complexes
All new ruthenium (II) complexes of composition [RuCl(CO)(Ef3)2(QT)] (E = As/P) were prepared by the ligand substitution in benzene using precursor complexes and ligand reported in our previous method.12 The other ruthenium (II) complex of composition [Ru(Pf3)2(QT)2] was prepared by direct reaction of [RuCl2(Pf3)4] with ligand (QTH) following our previous method.13
Analysis
[RuCl(CO)(Pf3)2(QT)] (yellow) : Calculated (%) for RuC45H35N2O2P2SCl (865.5) : C = 62.39; H = 4.04; N = 3.23; Cl = 4.10; Ru = 11.66; Found (%) : C = 62.42; H = 4.10; N = 3.33; Cl = 4.21; Ru = 11.55;
[RuCl(CO)(Asf3)2(QT)] (yellow) : Calculated (%) for RuC45H35N2O2As2SCl (953.5) : C = 56.63; H = 3.74; N = 2.99; Cl = 3.72; Ru = 10.59; Found (%) : C = 56.11; H = 3.77; N = 3.01; Cl = 80; Ru = 10.32;
Preparation of new ruthenium (III) complexes
All ruthenium complexes were prepared by following our previous method reported in literature.14
[RuCl2(Pf3)2(QT)] (Brown) : Calculated (%) for RuC44H35N2OSP2Cl2 (873) : C = 60.48; H = 4.00; N = 3.20; Ru = 11.56; Found (%) : C = 60.47; H = 4.11; N = 3.21; Ru = 11.66;
[RuCl2(Asf3)2(QT) (Brown) : Calculated (%) for RuC44H35N2OAs2Cl2 (961) : C = 54.94; H = 3.64; N = 2.91; Cl = 7.38; Ru = 10.50; Found (%) : C = 55.01; H = 3.66; N = 3.01; Cl = 7.48; Ru = 10.55;
[RuBr2(Pf3)2(QT)] (Brown) : Calculated (%) for RuC44H35N2OP2Br2 (962) : C = 54.88; H = 3.62; N = 2.91; Ru = 10.49; Found (%) : C = 54.98; H = 3.71; N = 3.01; Ru = 10.50 The analysis of C, H and N were performed at CDRI, Lucknow, India. The IR spectra of ligand and complexes were recorded on a Perkin-Elmer Model-577 Spectrophotometer in the range of 4000-200 cm-1 as KBr pillets. The magnetic measurements were made on a Gouy balance and diamagnetic corrections for the ligand molecule were applied. Electronic spectra were recorded with Beckmann DU-6 spectrophotometer. The molar conductance of complexes (10-3 M) were measured in DMF using Wiss-Wekstatter Weighein obb type LBR conductivity meter. The 1H NMR spectra were recorded on Bruker 400 MHz using TMS as reference.
Formula 1,2,3
The analytical data is consistent with the proposed stoichiometeries of the complexes. In all the above reactions, the thioamide ligand behaves as mononegative bidentate ligand. All isolated solid products are air stable in the solid state at room temperature and are non-hygroscopic in nature. All synthesized complexes are soluble in DMF, DMSO and acetonitrile producing intense coloured solution. The molar conductance in DMF (10-3 M) are found in the range of 10.16 – 15.5 Ù-1cm2mol-1 indicating their non-electrolytic nature.15 The chloride or bromide ion in corresponding complexes are present in the inner sphere and coordinated nature.
Magnetic Moment and Electronic Spectra
All ruthenium (II) chelates are diamagnetic indicating the ground state 1A1g arising from T2g6eg0 configuration in octahedral structure.16 The excited state corresponding to the T2g5eg1 configuration are 3T1g, 3T2g, 1T1g and 1T2g. Hence, four bands corresponding to the transition, 1A1g ® 3T1g, 1A1g ® 3T2g, 1A1g ® 1T1g and 1A1g ® 1T2g are possible in order of increasing energy. The electronic spectra [RuCl(CO)(Ef3)2(QT)] (E = P/As) exhibits bands at 21740 cm-1 (1A1g ® 1T1g), 23255 cm-1 (T2g ® p*, LMCT), 29850 cm-1 (n ® p*, LC) and 33890 cm-1 (p ® p*, LC) and 33890 cm-1 (p ® p*, LC) similar to those observed for the low-spin (Ru++, d6) complexes containing N,S-chelating thioamide, Pf3 or Asf3 and chloride ligands.17-19 The magnetic moment of [RuCl2(EΦ3)2(QT)] (E = P/As) chelates are found between 1.85 – 1.95 BM corresponding to one unpaired electron in T2g5 (Ru+++) in octahedral environment.20 The ground state of Ru (III) in octahedral environment is 2T2g arising from T2g5eg0 configuration. Hence, two bands corresponding to 2T2g ® 2A2g and 2T2g ® 2T1g are possible. The complexes display bands at 15870 cm-1 (2T2g ® 2A2g), 21970 cm-1 (LMCT) and 33115 (p ® p*) which indicates octahedral environment around ruthenium (III) ion and consistent with the other ruthenium (III) octahedral complexes.21
IR Spectra
A comparison of the IR spectra (table 1) of the ligand (QTH) and ruthenium (II & III) complexes brings out the following facts to light The medium broad nN-H bands of the ligand at 3446 and 3350 cm-1 are severely affected on coordination. The 3446 cm-1 band almost disappears indicating displacement of N-H hydrogen by means of Ru(II)/Ru(III) ion and formation of Ru(II)-N/Ru(III)-N bond. While the 3350 cm-1 band shift to lower frequency and gets split. (ii) The nC=O band of the ligand at 1710 cm-1 is found to be almost at the same place in the spectra of complexes indicating absence of bonding through carbony oxygen atom.22 (iii) All carbonyl complexes of ruthenium (II) display a new strong band in the region 1955-1960 cm-1 which is attributed due to terminally coordinated carbonyl group and is observed at higher frequency than in the precursor complexes.23 (iv) Thioamide bands24-26 I, II, III and IV are observed at 1530 (s), 1295 (m), 960 (m) and 790 (m) cm-1 in the spectrum of ligand (QTH). Band I splits, Band II blue shift to higher frequency, while band III and band IV red shift with reduction in intensity. (table 1) on complexation indicating simultaneous bonding with N and S atoms of QTH considering our previous observations.27-29 (v) New bands around 535, 685, 740 and 1560 cm-1 due to coordinated Asf3 and around 540, 690, 740 and 1535 cm-1 due to coordinated Pf3 molecule are in agreement with previous literature.30-32 (vi) The single Ru(III)-Cl stretching mode in [RuCl2(Ef3)2(QT)] indicates two mutual chlorine atoms are at Trans-position in octahedral structure (Str. III). Thus, the thioamide ligand acts as N, S-chelating mononegative bidentate nature in octahedral structure.
Table – 1 : Major IR Spectral bands (cm-1) of ligand (QTH) and complexes
|
Compounds |
nCºO |
Thioamide Bands |
nRu-N/ |
|||
|
|
|
|
nRu-S |
|||
|
Band I |
Band II |
Band III |
Band IV |
|||
|
QTH (ligand) |
– |
1530 (s) |
1295 (m) |
960 (m) |
790 (m) |
– (-) |
|
[RuCl(CO)(Pf3)2(QT)] |
1565 (m) |
1535 m |
1310 m |
955 w |
780 w |
455 m |
|
1510 m |
(420 w) |
|||||
|
[RuCl(CO)(Asf3)2(QT)] |
1566 (s) |
1535 m |
1315 m |
950 w |
780 w |
465 m |
|
1515 m |
(425 w) |
|||||
|
[RuCl(CO)(Pf3)(Py)(QT)] |
1570 ms |
1530 m |
1315 m |
950 w |
780 w |
470 m |
|
1515 m |
(420 w) |
|||||
|
[RuCl2(Pf3)2(QT)] |
– |
1530 m |
1310 ms |
945 vw |
780 w |
465 m |
|
1510 m |
(425 w) |
|||||
|
[RuCl2(Asf3)2(QT)] |
– |
1535 m |
1310 m |
945 vw |
785 w |
460 m |
|
1515 m |
(410 m) |
|||||
|
[RuBr2(Pf3)2(QT)] |
– |
1535 m |
1315 m |
950 w |
780 w |
455 m |
|
1510 m |
(415 w) |
|||||
|
[Ru(Pf3)2(QT)2] |
– |
1530 m |
1316 m |
950 w |
775 w |
465 m |
|
1515 m |
(420 w) |
|||||
1H NMR Spectra
1H NMR spectra of ligand and some complexes (Sl. No. 1, 2 & 3) were recorded in CDCl3/TMS to substantiate further metal-ligand bonding. The aromatic protons signals are observed at d7.8 – 8.6 PPM and the aromatic proton at position -5 is deshielded by carbonyl oxygen so the extreme signal at d8.3 PPM is considered to be due to this proton. The imino protons of the ligand observed at d3.2 – 3.6 PPM found to be absent in complexes indicate deprotonation of imino group. The 1H NMR spectra of complexes show a multiplet around d6.2-7.7 PPM which is assigned to aromatic protons of Pf3/Asf3.33 The resonances in the region d7.68 PPM, d8.2 PPM and d8.8 PPM assignable to the protons of Pyridine ligand along with the resonances due to aromatic protons in complexes.34 Thus, on the basis of physico-chemical, conductometric, magnetic, UV-vis, IR and 1H NMR data octahedral structure of both Ru(II) and Ru(III) complexes may be reasonably assigned.
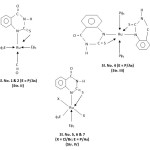 |
Scheme :2 Click here to View Scheme |
ACKNOWLEDGEMENT
One of the authors (KVG) gratefully acknowledge UGC (New Delhi) to award Junior Research Fellowship (JRF, NET) and thanks to Principal, Dr. R.K. Verma, College of Commerce (M.U.), Patna-800020 for necessary facilities.
REFERENCES
- Singh B., Rukhaiyar M.M.P. and Sinha R.J., J. Inorg. Nucl. Chem. Vol. 39, 29 (1977).
- Pandey R.N., Sinha Anil Kumar, Sharma R.N. and Ranjan R.K., Asian J. Chem. Vol. 6(2), 246 (1994).
- Pandey R.N., Sharma R.N., Choudhary L.M. Roy and Sharma Pramila, J. Indian Chem. Soc. Vol.69, 719 (1992).
- Dave G.R., J. Indian Chem. Soc. 37, 695 (1960).
- Stephenson T.A. and Wilkonson G., J. Inorg. Nucl. Chem. 28, 945 (1966).
- Ahmed N., Levision J.J., Robinson S.D. and Uttley M.F., Inorg. Synth, 15, 48 (1974).
- Delgade R.A.S., Lee W.Y., Choi S.R., Cho Y. and Jan M.Y., Transition Met. Chem. 16, 241 (1991).
- Balasubramanian K.P., Manivannan S. and Chinnusamy V., J. Ultra Chem. 4, 15 (2008).
- Chatt J., Leigh G.J., Mingos D.M.P. and Pask R.J., J. Chem. Soc. A, 2636 (1968).
- Natarajan K., Poddar R.K. and Agarwala U., J. Inorg. Nucl. Chem. 39, 431 (1997).
- Poddar R.K., Khullar I.P. and Agarwala U., J. Inorg. Nucl. Chem. Lett. 10, 221 (1974).
- Pandey R.N., Bala Renu and Sinha Anil Kumar, Oriental J. Chem. Vol. 27 (1), 293 (2011).
- Pandey R.N., Pandey Prashasti and Sharma R.N., Acta Ciencia Indica, Vol. 35 C(2), 217 (2009).
- Pandey R.N., Nag A.K. and Sharma D.K., Oriental J. Chem. Vol. 28(4), 1809 (2012).
- Geary W.J., Coord. Chem. Rev. 7, 81 (1971).
- Pandiarajan D. and Ramesh R., J. Organomet. Chem. 723, 26 (2013).
- Raja M. Ulaganatha and Ramesh R., J. Organomet. Chem. 699, 5 (2012).
- Murali S., Sastri C.V. and Maiya B.G., Proc. Indian Acad. Sci (Chem. Sci.) Vol 114(4), 403 (2002).
- Prabhu R.N. and Ramesh R., J. Royal Soc. Chem. 2, 4515 (2012).
- Raja N. and Ramesh R., Spectrochimica Acta, Part A, 75, 713 (2010).
- Samanta R., Mandal B., Munshi P. and Lahiri G.K., J. Chem. Soc. Dalton Trans, 1827 (2001).
- Pandey R.N. and Das J.N., J. Indian Chem. Soc. Vol. 71, 187 (1994).
- Chandra M., Sahay A.N., Pandey D.S., Puerta M.C. and Valerga Pedro, J. Organomet. Chem. 648, 39 (2002).
- Rao C.N.R. and Venkataraghavan R., Spectrochim. Acta, 18, 541 (1962).
- Rao C.N.R., Venkataraghavan R. and Kasturi T.R., Can. J. Chem. 42, 36 (1964).
- Suzuki I., Bull. Chem. Soc. (Japan) 35, 1286, 1449, 1456 (1962).
- Singh B., Pandey R.N., Sharma D.K., Sharma U.S. Pd. and Bhanu Uday, Indian J. Chem, 20A, 1097 (1982).
- Pandey R.N., Sharma Pramila and Kumar Manoj, J. Ultra Chem. Vol.9(2), 279 (2013).
- Pandey R.N., Aanand A., Singh R.K. and Kumar A., Asian J. Chem. Vol 22(7), 5601 (2010).
- Cao M., DO L.V., Hofmann N.W., Kwan M.L., Little J.K., Gilvray J.M. Mc, Morris C.B., Soderborg B.C. and Weirzbicki, Organometallics, 20, 2270 (2001).
- Kumar K.N. and Ramesh R., Polyhedron, 24, 1885 (2005).
- Shobalake K., Postmus C., Ferraro J.R. and Nakamoto K., Appl. Spectrosc. 23, 12 (1969).
- Balasubramian K.P., Raju V.V. and Chinnusamy V., J. Indian Chem. Soc. Vol.86, 570 (2009). Sisodiya S.S., Sahay A.N. and Pandey D.S., Indian J. Chem. 39A, 454 (2000).

This work is licensed under a Creative Commons Attribution 4.0 International License.










