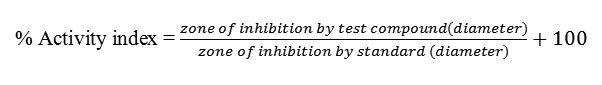Microwave Synthesis and Antimicrobial Activity of some Copper (II), Cobalt (II), Nickel (II) and Chromium (III) Complexes with Schiff Base 2, 6-Pyridinedi carboxaldehyde-Thiosemicarbazone
1Dr.Mohammed.Fakruddin Ali Ahmed, V. MahammadYunus
1Department of chemistry, College of Natural Science , Jimma University, Post-Box No:378
DOI : http://dx.doi.org/10.13005/ojc/300114
Article Received on : January 10, 2014
Article Accepted on : February 06, 2014
Article Published : 30 Mar 2014
Some novel Schiff base metal complexes of Cr(III), Co(II), Ni(II) andCu(II) derived from 2, 6-pyridinedicarboxaldehyde-Thiosemicarbazone(PDCTC) was synthesized by conventional as well as microwavemethods. This compound wascharacterized by elemental analysis, FT-IR, Mass, molar conductanceand magneticsusceptibilitymeasurements analyses. Analytical data revealed that all the complexesexhibited 1:1 (metal: ligand) ratio with a coordination number of six.The IR data showed that the ligand coordinates with the metal ions in ahexa-dentate manner. The solid state electricalconductivity of the metal complexes was also measured. Solid state electricalconductivity studies reflected a semi-conducting nature of the complexes. The Schiff base and metal complexes displayed good activity againstthe Gram-positive bacteria Staphylococcus aureus, the Gram-negative bacteriaEscherichia coli and the fungi AspergillusnigerandCandida albicans. The antimicrobialresults also indicated that the metal complexes displayed betterantimicrobial activity as compared to the Schiff bases.
KEYWORDS:microwave method; hexa-dentate ligands; biologicalactivities.
Download this article as:| Copy the following to cite this article: Ahmed A. F. M, Yunus M. V. Microwave Synthesis and Antimicrobial Activity of some Copper (II), Cobalt (II), Nickel (II) and Chromium (III) Complexes with Schiff Base 2, 6-Pyridinedi carboxaldehyde-Thiosemicarbazone. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Ahmed A. F. M, Yunus M. V. Microwave Synthesis and Antimicrobial Activity of some Copper (II), Cobalt (II), Nickel (II) and Chromium (III) Complexes with Schiff Base 2, 6-Pyridinedi carboxaldehyde-Thiosemicarbazone. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2565 |
Introduction
Microwave irradiation now a day is an accepted tool for accelerating the organic and inorganic reactions. It leads to the higher reaction selectivity and utilization of the inexpensive reagents. In addition to providing an eco-friendly “green chemistry” approach to the reaction, it is free of environmental impacts (1-4).the application of microwave irradiation towards the acceleration of wide range of organic and inorganic reactions has received concealable attention (5-10) .It also allowed a greener approach (11). Schiff base of an important class of ligands in coordination chemistry and have many applications (12), in different fields. The chemistry of Schiff base complexes continues to attract many researchers(13,14)because of their wide application in food industry, dye industry ,analytical chemistry catalysis ,antimicrobial activity, agro-chemical activity(15) and pharmacological applications(16).semicabazones of aromatic and unsaturated carbonyl compounds have anticonvulsant properties and their advantage over the analogous Thiosemicarbazone is their lesser neurotoxicity(17).Semicabazones have an inhibitory effect on nitric oxide synthesis, which protect the vascular system(18). It is well known that various organic ligands possess strong antibacterial, herbicidal, insecticidal and fungicidal properties (19).It has also been reported that the activity of bio metals is very often altered through the formation of chelates with different biological relevant ligands (20-23).It is suggest that the compounds having antimicrobial activity may act either by killing the microbe or blocking their active sites (24-26).In addition to this the antimicrobial activity of the compounds also depends upon the nature of the microorganisms.
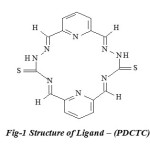 |
Fig-1 Structure of Ligand – (PDCTC) |
Materials and Methods
All the chemicals used were of AR grade and used without further purification. The infrared spectra were recorded in the range 4000-180 cm-1 with a Perkin Elmer 983 G spectrophotometer. The electronic spectra were recorded with Cary model 2390 spectrometer. The molar conductance of complexes in DMF (~ 10-3 M) was determined at 27± 20 C using a Systronic 303 direct reading conductivity bridge. The magnetic susceptibility measurements were made using a vibrating sample magnetometer (VSM) operating at field strength of 5 KG. The 1H NMR spectra was recorded on varian XL-300 MHz high resolution instrument in CDCl3 solvent. The mass spectra were recorded using Fanning Mat 8230 Mass spectrometer. Microwave assisted synthesis were carried out in open glass vessel on a modified microwave oven model 2001 ETB with rotating tray and a power source 230V, microwave energy output 800W and microwave frequency 2450MHz. A thermocouple device was used to monitor the temperature inside the vessel of the microwave. The microwave reactions were performed using on/off cycling to control the temperature.
Conventional method for the synthesis of Schiff bases
The reaction mixture containing 2, 6-Pyridinedicarboxaldehyde, (2g, 0.01183mol in 20ml of methanol) Thiosemicarbazone (1.0787g, .0.01183 mole in 20ml of methanol dissolved in hot condition) was taken in250-ml round bottom flask and refluxed for 10h. On cooling the reaction mixture, light yellow coloredproduct was formed. It was collected by filtration and washed with hot water and 50 % cold methanol. This compound was recrystallized from ethanol and dried in vacuo, yield 65% ; m. p. 257°C.(figure-2)
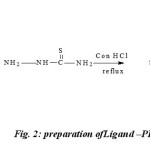 |
Fig. 2: preparation ofLigand –PDCTC |
Microwave method for the synthesis of Schiff bases
The equimolar (1:1) ratio of methyl isobutyl ketone with 2, 6-Pyridinedicarboxaldehyde, and Thiosemicarbazonewith isonicotinic acid hydrazide were mixed thoroughly in agrinder. The reaction mixture was then irradiated by the microwave oven by taking 3-4mL of dry ethanol as a solvent. The reaction was completed in a short time (4-5min) with higher (light yellow) yields. The resulting product was then recrystallized with ethanol, finally dried under reduced pressure over anhydrous CaCl2 in a desiccator. The progress of the reaction, purity of the product was monitored by TLC using silica gel G (yield: 85%).
Conventional method for the synthesis of metal complexes
The metal complexes (Figs. 3) was prepared by the mixing of equal moles of metal salts dissolved in the methanol was added followed by 1 ml of 1M NaOAc was added, in1:1 (metal: ligand) ratio. The resulting mixture was refluxed on water bath for 6- 8h. A coloured product appeared on standing and cooling the above solution. The precipitated complex was, filtered washed with ether and recrystallized with ethanol several times and dried under the reduced pressure over anhydrous CaCl2 in a desiccator. It was further dried in electric oven at 50-70°C (yield: 65-70%).
Microwave method for the synthesis of metal complexes
The ligand and the metal salts was mixed in 1:1 (metal: ligand) ratio in a grinder. The reaction mixture was then irradiated by the microwave oven by taking 3-4mL of dry ethanol as a solvent. The reaction was completed in a short time (5-9min) with higher yields. The resulting product was then recrystallized with ethanol and ether and finally dried under reduced pressure over anhydrous CaCl2 in a desiccator. The progress of the reaction and purity of the product was monitored by TLC using silica gel G (yield: 80-85%).
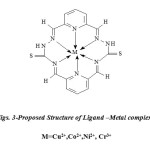 |
Figs. 3-Proposed Structure of Ligand –Metal complexes M=Cu2+,Co2+,Ni2+, Cr3+ |
Results and Discussion
The analytical data for all the complexes are given in Table-1. The molar conductivity data(Table-3) of the complexes are consistent with the non-electrolytic nature (28, 29)of the complexes. The ligand and complexes were characterized by elemental analysis to determine percentage of C, N, S and H. The observed and calculated percentages of the elements are in good agreement and support one ligand to a metal ion. The number of coordinated ligands to metal determined by Job’s continuous method and Mole ratio method established 1: 1 metal to ligand ratio.
Table-1:The comparative results of conventional and microwave methods–Analytical Data of PDCTC and their metal complex
|
Compound / complex (colour) |
M.Pt.0C
|
Reaction period |
Yield % |
Mol. Wt. |
Elemental Analysis Found (calculated) |
||||||
|
CMM(h.) |
MM (min.) |
CM |
MM |
C % |
H% |
N% |
S% |
M% |
|||
| PDCTC(light-yellow colour) |
257 |
10 |
5 |
65 |
84 |
380.5 |
50.4 |
3.1 |
29.4 |
16.8 |
– |
|
(42.3) |
(2.6) |
(24.7) |
(14.1) |
– | |||||||
| PDCTC -Co(Light brown colour) |
215 |
8 |
9 |
70 |
85 |
439.4 |
43.6 |
2.7 |
25.4 |
14.5 |
13.4 |
|
(37.1) |
(2.3) |
(21.6) |
(12.3) |
(11.3) |
|||||||
| PDCTC -Cu(black colour ) |
210 |
6 |
8 |
55 |
84 |
444 |
43 |
2.7 |
25.2 |
14.4 |
14.3 |
|
(36.2) |
(2.2) |
(21.1) |
(12.1) |
(12) |
|||||||
| PDCTC -Ni (light green colour ) |
230 |
8 |
5 |
61 |
85 |
439.4 |
43.6 |
2.7 |
25.4 |
14.5 |
13.3 |
|
(37.1) |
(2.3) |
(21.6) |
(12.3) |
(11.3) |
|||||||
|
PDCTC -Cr (yellowish green colour ) |
270 |
7 |
6 |
64 |
82 |
432.5 |
44.3 |
2.7 |
25.8 |
14.7 |
12 |
|
(36.4) |
(2.2) |
(21.2) |
(12.1) |
(9.8) |
|||||||
IR and 1H NMR Spectral Analysis
The reagents have been characterized by IR and 1H NMR spectral data. The infrared spectra of PDCTC show bands at 1697 cm-1 for ѴC=N; 722 for ѴC-S; 1540 for ѴC=S; indicating the Schiff base formation. The lowering of ѴC=N of azomethine group to the extent of 30-50 cm-1 in all the complexes suggests the participation(27-29)of azomethine nitrogen in complexation. On coordination, this band is shifted to lower frequency suggests that the ligand is coordinated to metal ion via azomethine nitrogen in all complexes. This change in shift is due to the drift of the lone pair density of azomethine nitrogen towards metal atom (30). In the far IR spectral region, additional medium to strong bands at 405-420 and 325-355 cm-1 are assigned to ѴM-N and ѴM-S modes(31,32)respectively.1H NMRspectra of PDCTC (CDCl3 + DMSO-d6) showed signals at 2.27, (1H,s); 8.15-8.32(1H),7.10,7.86(4H,s) 3.25(1Hs)due to C=N(C5H4N),NH. The magnetic moment(Table-4) value of Cu-PDCTC was 2.24 BM indicates one electron paramagnetism. This value is higher than the spin-only value of 1.73 BM for one unpaired electron. The higher value of the magnetic moment indicates that complexes are monomeric in nature and there is no metal-metal interaction along the axial position in the complex and have distorted octahedral environment(33-35). The magnetic moment of Co-PDCTC was found to lie in 2.05 BM. Monomeric cobalt complexes have lower magnetic moment values than would be expected for pure octahedral complexes suggesting flattening towards planar arrangement (36-40).The magnetic moments of Ni (II) complex was observed at 3.73 BM. This value is in the range reported earlier for octahedral complexes (41).Cr(III) complexwas observed at 2.12 BM.
Table 2: Selected IR bands (cm-1) with tentative assignments
| Compound |
ѴC=N
|
ѴC-S
|
ѴC=S
|
ѴM-N
|
ѴM-S
|
|
PDCTC |
1697 |
722 |
1540 |
– |
– |
|
Cu-PDCTC |
1615 |
650 |
1560 |
420 |
355 |
|
Co- PDCTC |
1608 |
708 |
1550 |
415 |
352 |
|
Ni- PDCTC |
1610 |
707 |
1540 |
412 |
340 |
|
Cr- PDCTC |
1623 |
712 |
1545 |
405 |
325 |
Table 3: Molar conductance data of metal complexes of PDCTC
|
PDCTC – Complex |
Conductance(Ohm-1Cm2mol-1) |
|
Cu-PDCTC |
26 |
|
Co- PDCTC |
24 |
|
Ni- PDCTC |
22 |
|
Cr- PDCTC |
36 |
Table 4: Magnetic moment data of metal complexes of PDCTC
|
PDCTC– Complex |
Magnetic Momentum(B.M) |
|
Cu- PDCTC |
2.24 |
|
Co- PDCTC |
2.05 |
|
Ni- PDCTC |
3.73 |
|
Cr- PDCTC |
2.12 |
Antimicrobial activities
The in-vitro Antimicrobial activity of the synthesized Schiff base ligands and their corresponding metal complexes on selected bacteria E. coli and S. aureusand two fungi A. nigerandC. albicanswas carried out. All of the tested compounds showed good biological activity against microorganism. On comparing the biological activity of the Schiff base and its metal complexes with the standard bactericide and fungicide, it is show that the some metal complexes have good activity as compared to the standard but all the complexes are more active than their respective ligands. The higher inhibition zone of metal complexes than those of the ligands can be explained on the basis of Overtone’s concept and Chelation theory. On chelation, the polarity of the metal ion will be reduced to greater extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor groups. Further, it increases the delocalization of π-electrons over the whole chelating ring and enhances the penetration of the complexes into lipid membranes and blocking of the metal binding sites in the enzymes of microorganisms. There are other factors which also increase the activity are solubility, conductivity and bond length between the metal and ligand(42-45).The bactericidal and fungicidal investigation data of the compounds are summarized in Tables 5 and 6. The results of the investigations account for the anti-pathogenic behavior of the compounds and this efficacy is positively modified on complexation.
Table 5: Antibacterial screening data for the ligands and their complexes
|
Compound |
E .coli |
S. aures |
|||||||||||
|
Diameter of inhibition zone(mm) |
% Activity index |
Diameter of inhibition zone(mm) |
% Activity index |
||||||||||
|
25 |
50 |
100 |
25 |
50 |
100 |
25 |
50 |
100 |
25 |
50 |
100 |
||
|
PDCTC |
10 | 15 | 18 | 45 | 60 | 62 | 12 | 16 | 19 | 63 | 64 | 76 | |
| Cu-PDCTC | 13 | 16 | 20 | 59 | 64 | 68 | 13 | 15 | 20 | 68 | 71 | 80 | |
| Co-PDCTC | 14 | 17 | 21 | 63 | 68 | 72 | 12 | 14 | 18 | 63 | 66 | 72 | |
| Ni-PDCTC | 16 | 19 | 23 | 72 | 76 | 79 | 11 | 15 | 18 | 57 | 71 | 72 | |
| Cr-PDCTC | 17 | 20 | 24 | 77 | 80 | 82 | 10 | 16 | 20 | 52 | 76 | 80 | |
|
22 | 25 | 29 | 100 | 100 | 100 | 19 | 21 | 25 | 100 | 100 | 100 | |
Table 6: Antifungal screening data for the ligands and their complexes
|
Compound |
Diameter of inhibition zone (mm); Concentration in ppm |
|||||
|
A .nizer |
C.albicans |
|||||
|
25 |
50 |
100 |
25 |
50 |
100 |
|
|
PDCTC |
12 |
15 |
21 |
13 |
16 |
20 |
| Cu-PDCTC |
14 |
18 |
23 |
14 |
19 |
24 |
| Co-PDCTC |
15 |
20 |
24 |
15 |
18 |
22 |
| Ni-PDCTC |
16 |
19 |
23 |
14 |
17 |
21 |
| Cr-PDCTC |
15 |
21 |
25 |
18 |
20 |
25 |
|
||||||
Conclusion
In the present research studies, our successful efforts are synthesis of some newly compounds from the conventional as well as microwave methods. These synthesized compounds have been characterized by various physicochemical,VSM and spectral analyses. In the result of microwave-assisted synthesis, it has been observed that the reaction time decreased from hours to minutes and availability of the product within better yields compared to the classical method. Electrical conductivity data suggest that all the complexes fall in the semiconducting range. The antimicrobial data show that the metal complexes to be more biological active compared to those parent Schiff base ligand against all pathogenic species. The compounds also inhibit the growth of fungi and bacteria to a greater extent as the concentration is increased. The Schiff base ligands were found to be biologically active and their metal complexes displayed enhanced antimicrobial activity against one or two strains. Chelation tends to make the ligand act as more powerful and potent bactericidal agent. Further chelation can help in MDR problems.
References:
- Ashry,E S H E1,Ramadan E,Kassem E, Kassem A A& Hager M,AdvHeterocycl Chem,68(2005)1.
- Kappe C O&Loupy A ,Microwave in Organic Synthesis (Wiley-VCH,Weinheim),2002,pp405.
- Kappe C O,Curr Opinion Chem Bio,6 (2002)314.
- Danida A, Arya K, Sati M &Gautam S, Tetrahedron,60 (2004) 5253.
- Gedye R, Smith F,Westaway K, Ali H, Baldisera L, Laberge L &Rousell J, Tetrahedron Lett., 27 (1986) 279.
- Gedye R J, Bray T L & Duncan S M, Tetrahedron Lett., 27 (1986) 2945.
- CaddickS,TetrahedronLett., 50 (1994) 10403.
- Mingos D M P &Baghurst D R, Chem Rev,20(1991)1.
- Whittaker A G &Mingos D M P,J Microwave Power Electomag Energy, 29 (1994)195.
- Mingos D M P,ResChemIntermid, 20 (1994) 85.
- Whittaker A G,EducChem,(2002) 134.
- Suma S, Kumar M R S,Nair C R &Prabhakaran C P, Indian J Chem,32A (1993)214.
- Warad D U, Salish C D, Kulkarani V H &Bajgur C S,Indian J Chem,39A (2000)415.
- Sen A K, Singh G, Singh K, Noren R K, Handa R N &Dubey S N,Indian J Chem,36A (1997)891.
- Ramarao N, Rao P Venkateduwar, Reddy G Venkat&Gasnorkar M C,Indian J Chem,26A (1987)887.
- Santoskar R S &Bhandarkar S D,Pharmacology and Pheumacothepeutic (Popular Prakasan, Bombay),1993,pp.648.
- Dimmock J R, Vashishta S C & Stables J P, Eur J Med Chem,331 (200)241.
- Sogani P, Yang S, Pillette C, MureawR,Gadano, Arenwel G, Bloy C &LebeacD,Eur J Pharmacol, 37(1998)344.
- Maurya R C, Mishra D D, Trivedi P K & Gupta A, Synth React Inorg Met Org Chem 17(1994) 24.
- Rainsford K D &Witchose M W, J Pharma Pherimacol,28(1976) 83.
- Sharma R C, Parashar R K &Mogan G, J Biol Trace Element Res,23(1990) 145.
- Sharma R C&Varshney V K, J InorgBiochem,(1991) 228.
- Raman N, Kulandaisamy A &Thangaraja C,T rans Met Chem, 28(2003) 29.
- Rao D S &Gonorkar M C,J Indian ChemSoc, 58(1981) 217.
- Athar M,Ahmad N, Gupta A A&Sengupta A K, Indian Drugs (1985) 225.
- Cghoi Y K, Choi K H, Pai S M &Dodapanenil N, J Electrochem Soc,142(1995)4107.
- Srivastava S K, Pandya K.P & Nigam H L, Indian J Chem, 12 (1974) 530.
- Ahmed, A and Akhtar F (1983): Indian Jour. Chem. 20 A, pp 737-758
- Byeong-Goo J., Chae-Pyong R., Hee-Nam c, Ki-Hyung C. and Yohng-Kook C (1996) Korean chem.. Soc. Vol 17, no. 8, pp 687-693
- Sarika R et al. Am-Eura J Sci Res 2009; 4 (4): 229-234
- Sinn E & Morris C M, Coordchem Rev, 4 (1969) 891.
- Nakagawa L &Shimanonchi T, SpecrochimActa, 20 (1964) 429.
- Joseph J., Nagashri K., AjishaBibin Rani G., Journal of Saudi chemical society 2011
- Sulekh Chandra, DeepaliJain ,AnjanaSarkar and Anupama J. Indian chem.. soc., vol.86. March 2009, pp 220-224.
- Zahid H. Chohan and Syed K.A. Sherazi Metal based drugs, Islamic university, Bahawalpur, Pakistan Figgis BN & Lewis J, ProgsInorg chem. 6 (1964) 37
- Hussain Reddy K., Radha Krishna Reddy M., &Lingappa Y., Indian Journal of chem… vol. 35 A, 9 (1996) 775-778
- Bottcher A., Elias H., E-G. Jager, Langfelderova H., Mazur M., Muller L., Paulus H., Pelikan P., Rudolph M., Valko M., Inorg. Chem… 1993, 32, 4131
- Huber A., Dr. Ing. Dissertation.TechnischeHochschule Darmstadt. 1997
- Green wood N. N., Earnshaw A., Chemie der Elemente, VCH, Weinheim. 1988
- Malik, W.U., Bembi, R. and Singh R., Trans Met. Chem.., 8, 321 (1983)
- Vinod Kumar and Rajesh Dhakarey; Journal of the Indian council of chemistry vol. 20, No.1 2003, pp 46-51
- Z Chohan, AMunawar, C Supuran. Transition metal ion complexes of Schiff bases synthesis, characterization and antibacterial properties. Metal Based Drugs,8, 137-143 (2001).
- W Hanna, M Moawad. Synthesis, characterization and antimicrobial activity cobalt(II), nickel(II) and copper(II) complexes with new asymmetrical Schiff base lagands derived from 7-formyanil- substituted diamine-sulphoxine and acetylacetone. Transition Metal Chem., 26(6), 644-651 (2001).
- J Iqbal, S Tirmizi, F Watto, M Imran. Biological Properties of Chloro-Salicylidene Aniline and its Complexes With Co(II) And Cu(II). Turk J. Biol., 30, 1-4 (2006).
- V Singh, AKatiyar. Synthesis, characterization of some transition metal(II) complexes of acetone p-amino acetophenonesalicyloylhydrazone and their antimicrobial activity. Bio Metals, 21(4), 491-501 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.