Graftig Copolymerization of Hydrophilic Monomers Onto Alginate for Modification it,s Structure
Mohammad Sadeghi*, Esmat Mohammadinasab, Fatemeh Shafiei, Laleh Mansouri And Hadis Shasavari
Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak, Iran,
DOI : http://dx.doi.org/10.13005/ojc/300130
Article Received on :
Article Accepted on :
Article Published : 06 Feb 2014
In this research, we synthesize of a graft copolymer based on alginate via simultaneously graft copolymerization acrylamide (AAm) and 2-acrylamido-2-methylpropanesulfonic acid (AMPS) onto alginate backbones. The polymerization reaction was carried out in an aqueous medium and in the presence of ammonium persulfate (APS) as an initiator. Infrared spectroscopy and TGA thermal analysis were carried out to confirm the chemical structure of the copolymer. A proposed mechanism for hydrogel formation was suggested.
KEYWORDS:alginate; graft ; copolymer; hydrophilic monomers
Download this article as:| Copy the following to cite this article: Sadeghi M, Mohammadinasab E, Shafiei F, Mansouri L, Shasavari H. Graftig Copolymerization of Hydrophilic Monomers Onto Alginate for Modification it,s Structure. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Sadeghi M, Mohammadinasab E, Shafiei F, Mansouri L, Shasavari H. Graftig Copolymerization of Hydrophilic Monomers Onto Alginate for Modification it,s Structure. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2035 |
Introduction
The modification of natural polymers is a promising method for the preparation of new materials(1). An efficient approach to modify of natural polymers, in order to synthesis of natural-based SAPs, is graft polymerization of vinylic monomers onto their backbones in the presence of crosslinkers. Free radical graft copolymerization with various monomers can carried out with different initiator systems[1-3]. The literature survey, however, reveals that few of the modifications deal with chemical grafting of a pre-modified polysaccaride such as alginate. APS-initiated grafting of vinyl monomers such as methyl acrylate, ethyl acrylate and ethyl methacrylate[4], AN/methyl methacrylate mixture, acrylamide (AAm)[5], and 4-vinylpyridine[6] onto alginate has been reported. However, to the best of our knowledge, no report has been published on the optimization graft polymerization of Acrylamide (AAm) and 2-Acrylamido-2-methyl propan sulfonic acid (AMPS) togather onto alginate chains using APS- alginate initiating system. In the present report, to modify the alginate, the grafting of Acrylamide (AAm) and 2-Acrylamido-2-methyl propan solfonic acid (AMPS) onto alginate chains in the presence of ammonium persulfate (APS) as an initiator was performed in a homogeneous system.
Experimental Materials
Sodium alginate (chemical grade, MW 50000), ammonium persulfate (APS, from Fluka), acrylamide(AAm, from Merck), 2-acrylamido-2-methylpropanesulfonic acid (AMPS, from Merck), were used without further purification. All other chemicals were also analytical grade. Double distilled water was used for the hydrogel preparation and swelling measurements.
Procedure to Graft Copolymerization
Synthesis of the copolymer, alginte-g-poly(AAm-co-AMPS), was carried out using APS as an initiator in an aqueous medium. A general procedure for graft copolymerization of two monomers together onto alginte was conducted as follows. alginate (0.20-0.80 g) was added to a three-neck reactor equipped with a mechanical stirrer (Heidolph RZR 2021, three blade propeller type, 300 rpm), including 40 mL doubly distilled water. The reactor was immersed in a thermostated water bath preset at desired temperature (45-85 oC). After complete dissolution of alginte, various amounts of the initiator solution (0.01-0.40 g APS in 5 mL H2O) were added to the mixture. After stirring for 10 min, certain amounts of AAm (0.8-2.2 g in 5 mL H2O) and AMPS (0.10-0.65 g in 5 mL H2O) monomers were simultaneously added to the reaction mixture. The mixture was continuously stirred at the desired temperature until completion of the reaction (2 h). After adding hydroquinon solution (0.5 wt%, 2 mL), cooling to room temperature, and adding few droplets of a molar sodium hydroxide solution to neutralize the remaining acid, the product was then precipitated in excess amount of methanol while mild stirring for ten minutes. The product was filtered, thoroughly washed with methanol and dried at 50 oC for one hour[5].
RESULTS AND DISCUSSION Mechanism of hydrogel formation
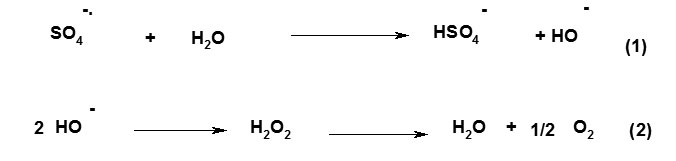
A general reaction mechanism for graft copolymerization of AAm and AMPS monomers onto alginate backbones in the presence of APS initiator is shown in Scheme 1. The sulfate anion-radical produced from thermally decomposition of APS, abstracts hydrogen from one of the functional groups in side chains (i.e. COOH, SH, OH, and NH2) of the substrate to form corresponding radical[6-9]. Then the resulted macroradicals radically initiate graft copolymerization of AAm and AMPS monomers led to a graft copolymer so called alginate-g-poly(AAm-co-AMPS).
 |
Scheme 1. Proposed mechanistic pathway for synthesis of Alginate-based copolymer. |
Results and Discussion
Grafting Evidences
The simplest method to prove the formation of alginate-g-poly(acrylamide-co-AMPS) is based on the solubility difference of the graft copolymer and the homopolymers, PAAm and PAMPS. alginate and homopolymers are soluble in water and DMF, respectively. When a reaction product was Soxhlet-extracted with DMF and alternately with water for 24h, an insoluble solid was still remained[10]. A alginate /PAAm-PAMPS physical mixture was dissolved completely when it was treated in the same was. Therefore, it is obvious that the graft copolymer obtained was not a simple physical mixture, but some chemical bonds must exist between the alginate substrate and PAAm-PAMPS macromolecules[11]. The PAAm-PAMPS grafting was also confirmed by the differences between FTIR spectra of the alginate and that of the graft copolymer. Figure 1 shows the FTIR spectra of the alginate substrate and the alginate-g-PAAm-AMPS graft copolymer freed from homopolymers. In Fig. 1a, the band observed at 1655 cm-1 can be attributed to C=O stretching in carboxamide functional groups of substrate backbone (Fig. 1a). The broad band at 3200-3600 cm-1 is due to stretching of –OH groups of the alginate. The existence of a rather sharp intense peak at 1280 cm-1 (Sulfonic groups) and1673 cm-1 (carboxamide groups) in IR spectra of the graft copolymers is a certain evidence of grafting . This absorption band arises from stretching vibration mode of the sulfonic and carboxamide groups related to acrylamide and 2-Acrylamido-2-methyl propan Sulfonic acid monomers respectively[12-14]. Most of the other peaks are related to the protein backbone. Since PAAm-AMPS could be extracted nearly completely from a physical mixture of PAAm, PAMPS and alginate by DMF, the presence of appreciable amounts of sulfonic and carboxamide groups in our reaction products after extraction is an additional proof for grafting of PAAm and PAMPS onto the alginate.
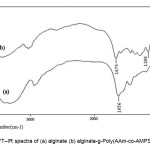 |
Figure 1. FT–IR spectra of (a) alginate (b) alginate-g-Poly(AAm-co-AMPS) copolymer
|
Thermogravimetric behavior
The grafting was also supported by thermogravimetric analysis (Fig. 2). TGA of alginate (Fig. 2a) shows a weight loss in two distinct stages. The first stage ranges between 17 and 145 oC and shows about 19% loss in weight. This may correspond to the loss of adsorbed and bound water. No such inflexion was observed in the TGA curve of alginate-g-P(AAm-co-AMPS). This indicated that the grafted copolymers were resistant to moisture absorption[15-16]. The second stage of weight loss starts at 230 oC and continues up to 300 oC during which there was 53% weight loss due to the degradation of alginate. Grafted samples, however, show almost different behavior of weight loss between 22 and 410 oC (Fig. 2b). The first stage of weight loss starts at 305 oC and continues up to 330 oC due to the degradation of alginate. The second stage from 365 to 450 oC may contribute to the decomposition of different structure of the graft copolymer. The appearance of these stages indicates the structure of alginate chains has been changed, which might be due to the grafting of poly(AAm-co-AMPS) chains. In general, the copolymer had lower weight loss than alginate. This means that the grafting of alginate increases the thermal stability of alginate in some extent[17].
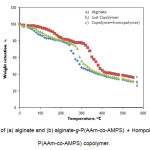 |
Figure 2. TGA curves of (a) alginate and (b) alginate-g-P(AAm-co-AMPS) + Hompolymers. (c) alginate-g-P(AAm-co-AMPS) copolymer. |
Optimization of Polymerization
Since polymerization variables determine the extent of grafting and homopolymer amount, certain factors affecting the grafting parameters were investigated to achieve the optimum condition of polymerization. Therefore, we optimized the grafting of two monomers togather onto alginate in homogenous aqueous media by changing temperature, the initial concentration of initiator, and the relative amount of the substrate. Within the range of the amount of the reactants used, our preliminary studies showed no considerable dependence between the reaction time and the grafting extent. The conversion as well as the grafting parameters, i.e. homopolymer content (Hp), grafting ratio (Gr) and the grafting add-on values was calculated using the following equations[8]:
Conversion= (W3-W0)/W1 (1)
Gr= W3/W0 (2)
Hp= W2/(W2+W3) (3)
Add-on= (W3-W0)/W3 (4)
Where W0, W1, W2 andW3 are weight of the initial substrate, the monomer charged, the homopolymer extracted, the homopolymer-free graft copolymer, respectively.
Effect of Initiator Concentration
The grafting dependence on APS concentration can be concluded Figure 3. The highest grafting ratio (97%) was achieved at 0.008 mol/L of APS where homopolymer content was 24.0 %. Increased APS concentration resulted in more radical sites on the alginate backbone that inturn led to higher Gr and add-on values and lower homopolymers formation. As a result, increased free radicals on alginate are compensated by partial termination of the macroradicals[11]. Thus Gr and add-on values were diminished at higher amounts of the initiator.
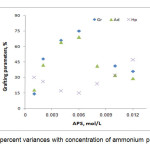 |
Figure3. Grafting percent variances with concentration of ammonium persulfate variance Click here to View Figure |
Effect of Reaction Temperature
To study the influence of the reaction bath temperature on the grafting parameters, the grafting of two monomers togather onto alginate was carried out at seven temperatures ranging from 35 to 85 oC. The results are given in Figure 4. Grafting percentage (%Gr) is increased with increasing the temperature from 35 to 75 oC, and then decreased. At 75 oC, maximum grafting (Gr 187.0 %), minimum homopolymer content (13.0%) was obtained. Improvement of grafting up to 75 oC can be attributed to the following factors: 1) increased the number of free radicals formed on the alginate backbone, 2) increased propagation of the graft copolymerization onto alginate, 3) enhanced diffusion of monomers and initiator into and onto backbone structure, and 4) increased in mobility of the monomers molecules and their higher collision probability with the backbone macroradicals. However, Gr was decreased as the bath temperature was raised beyond 75 oC. this can 1) be accounted for in terms of chain radical termination at higher temperatures. 2) Premature termination of growing chains and instability of the APS-alginate[10], are presumably another reasons for reduced amount of grafting beyond 75 oC. 3) oxidative degradation of alginate chains by sulfate radical-anions, 4) decomposition of APS to give O2 (a radical scavenger[8]), which reacts with primary free radicals (Eqs. 1 and 2), resulting in decreased molecular weight and decreased grafting (the sulfate radical anions may react with water to produce hydroxyl radicals (Eq. 1) and finally oxygen (Eq. 2)).
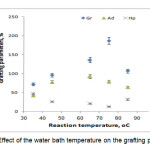 |
Figure 4. Effect of the water bath temperature on the grafting parameters. |
The rates graft copolymerization (Rg) may be evaluated as measures of the rate of monomers disappearance by using the following equations[13]: 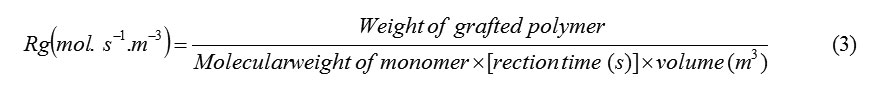
Overall activation energy of grafting (Ea) may also be estimated from the temperature data through plotting lnRg versus 1/T (oK-1) for the initial portion of the data of the temperature series given in above text. The slope of this Arrhenius plot (Figure 3) resulted in a rough estimation of Ea of grafting using the relationship slope = -Ea/R; where R is the universal gas constant. Therefore, Ea for the graft copolymerization was found to be 33.40 kJ/mole[10].
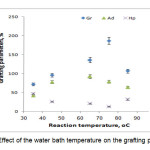 |
Figure 4. Plot of lnRg-1/T for estimating the activation energy of the graft polymerization reaction. |
Effect of alginate Concentration
The related to the grafting dependence on alginate amount is summarized in Figure 5. Maximum grafting(211%) and the lowest homopolymers formation was observed at 3wt% alginate, while others reactants including, monomers, initiator, and temperature were kept constant. Beyond this value, both grafting ratio and add-on values are considerably reduced. This behavior is attributed to the availability of more grafting sites for initiation of graft copolymerization at higher concentration of the substrate (until 3wt% alginate). However, upon further increase in the substrate concentration, increase in the reaction medium viscosity restricts the movements of macroradicals leading to decreased grafting ratio and add-on values. It also may be attributed to deactivation of the macroradical growing chains (e.g., by transfer reactions, combination and/or interaction with the primary radicals) soon after their formation[9,12].
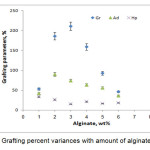 |
Figure 5. Grafting percent variances with amount of alginate variance |
CONCLUSIONS
A novel superabsorbent was prepared by graft copolymerization of 2-acrylamido-2-methylpropanesulfonic acid onto alginate in the presence of a crosslinking agent. The resultant superabsorbent composite had a large degree of water absorbency. The study of FTIR spectra shows that in the hydrogel spectrum a new absorption band at 11280 and 1364 cm-1 was appeared that attributed to S-O stretching in AMPS monomer grafted onto polysaccharide backbones. The effect of the individual factors was investigated by calculating the grafting parameters, i.e., grafting percent (Gr), add-on value and homopolymer content (Hp). Under optimum conditions (alginate 3wt%, APS 0.008 mol/L, reaction temperature 75 oC), the grafting parameters were achieved as 211%, 74 %, and 15% respectively. According to the slope of LnRg versus 1/T, the overall activation energy for graft copolymerization reaction was estimated to be 33.40 kJ/mole.
References
- Flory, P. J. In Principles of Polymer Chemistry, Cornell University press, New York: Ithaca, 1953.
- Mi, P.; Ju, X.; Xie, R.; Wu, H.; Ma, J.; Chu, L. Polymer, 51, 1648-1653(2010).
- Tang, Q.; Wu, J.; Lin, J. Carbohyd Polym, 73, 315-321(2008).
- George, M.; Abraham, T. E. Int J Pharma, 335, 123-129(2007).
- Sadeghi, M.; Hosseinzadeh, H. Turk J Chem, 32, 375-388(2008).
- Bajpai, A. K.; Giri, A. Carbohydr Polym 2003, 53, 271-278.
- Mahdavinia, G. R.; Pourjavadi, A.; Hosseinzadeh, H.; Zohuriaan, M. J. Europ Polym J, 40, 1399-1407(2004).
- Fanta, G. F. In Polymeric Materials Encyclopedia. Salamone, J. C., editor. Florida: CRC Press, Boca Raton, vol.10. p. 7901, 8051, (1996).
- Rathna, G.V.N.; Damodaran, S. J Appl Polym Sci, 85, 45-51(2002).
- Yan, H.; Saiani, A.; Gough, J. E.; Miller, A. F. Biomacromolecules, 7, 2776-2782(2006).
- Pourjavadi, A.; Kurdtabar, M.; Mahdavinia, G. R.; Hosseinzadeh, H. Polym Bull, 57, 813-824(2006).
- Barvic, M.; Kliment, K.; Zavadil, M. J. Biomed Mater Res, 1967, 1, 313–323.
- Chirila, T. V.; Constable, I. J.; Crawford, G. J.; Vijayasekaran, S.; Thompson, D. E.; Chen, Y. C.; Fletcher, W. A.; Griffin, B. J. Biomaterials, 14, 26–38(1993).
- Chen, J.; Park, K. J Control Rel65, 73-82(2000).
- Chen, J.; Park, K. Carbohydr Polym, 41, 259-268(2000).
- Gotoh, T.; Nakatani, Y.; Sakohara, S. J Appl Polym Sci, 69, 895-906(1998).
- Badiger, M. V.; McNeil, M. E.; Graham, N. B. Biomaterials, 14, 1059-1063(1993).

This work is licensed under a Creative Commons Attribution 4.0 International License.