Comparative Study of Adsorption Isotherms of Vitamin C on Multi wall and single wall Carbon Nanotube
Azin Dehmolaei1, Mehdi Vadi2*
1) Department of Chemistry,Gachsaran Branch, Islamic Azad University,Gachsaran, Iran
2) Department of Chemistry,Sarvestan Branch, Islamic AzadUniversity ,Sarvestan, Iran
DOI : http://dx.doi.org/10.13005/ojc/300128
Article Received on :
Article Accepted on :
Article Published : 27 Jan 2014
We have studied the interaction of Vitamin C solution on multi-wall and single-wall carbon nanotubeAfter investigated comparative study and assigned to Vitamin C adsorption isotherm. The adsorption equilibrium isotherms were fitted by Freundlich, Langmuir, and Temkin models. It was found that the Langmuirmodel described the adsorption process better than other two isotherm models. The amount of Antioxidant drug(Vitamin C) adsorbed on Multi wallcarbon nanotube surface increased with the increase of the initial Antioxidant concentration. Based on the results, under similar conditions the efficiency of adsorption of Vitamin C by Multi-wall carbon nanotube(MWCNTs) was more thansingle-wall carbon nanotube.
KEYWORDS:Adsorption, Isotherms, Antioxidant ; MWCNT
Download this article as:| Copy the following to cite this article: Dehmolaei A, Vadi M. Comparative Study of Adsorption Isotherms of Vitamin C on Multi wall and single wall Carbon Nanotube. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Dehmolaei A, Vadi M. Comparative Study of Adsorption Isotherms of Vitamin C on Multi wall and single wall Carbon Nanotube. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=1965 |
Introduction
Nanotubes are categorized as single walled nanotubes (SWNTs) and multi walled nanotubes (MWNTs)[1]. Individual nanotubes naturally align themselves into ropes held together by vanderwaals forces, more specifically, orbital hybridization best describes chemical bonding in nanotubes. The chemical bonding of nanotubes is composed entirely of sp2 bonds, similar to those of graphite. These bonds, which are stronger then the sp3 bonds found in alkanes and diamond, provide nanotubes with their unique strength. Multi walled nanotubes (MWNT) consist of multiple rolled layers (concentric tubes) of graphene. In the parchment model, a single sheet of graphite is rolled in around itself, resembling a scroll of parchment or a rolled newspaper. The Russian Doll structure is observed more commonly[2]. Its individual shells can be described as SWNTs, which can be metallic or semiconducting. Because of statistical probability and restrictions on the relative diameters of the individual tubes, one of the shells, and thus the whole MWNT, is usually a zero-gap metal.Double-walled carbon nanotubes (DWNT) form a special class of nanotubes because their morphology and properties are similar to those of SWNT but their resistance to chemicals is significantly improved. This is especially important when functionalization will break some c=c double bonds, leaving “holes” in the case of DWNT, only the outer wall is modified. DWNT synthesis on the gram-scale was first proposed in 2003 [3] by the by the CCVD technique, from the selective reduction of oxide solutions in methane and hydrogen. The telescopic motion ability of inner shells [4] and their unique mechanical properties [5] permit to use multi-walled nanotubes as main movable arms in coming nanomechanical devices. Retraction force that occurs to telescopic motion caused by the Lennard – Jones interaction between shells and its value is about 1.5 nN[6].An antioxidant is a molecule that inhibits the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When the chain reaction occurs in a cell, it can cause damage or death to the cell. Antioxidants terminate these chain reactions by removing free radical intermediates, and inhibit
other oxidation reactions. They do this by being oxidized themselves, so antioxidants are often reducing agents such as thiols, ascorbic acid, or polyphenols[7]
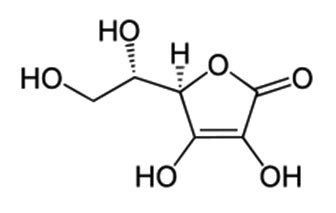
Vitamin C or L-ascorbic acid, or simply ascorbate (the anion of ascorbic acid), is an essential nutrient for humans and certain other animal species. Vitamin C refers to a number of vitamers that have vitamin C activity in animals, including ascorbic acid and its salts, and some oxidized forms of the molecule like dehydroascorbic acid.Vitamin C is a cofactor in at least eight enzymatic reactions, including several collagen synthesis reactions that, when dysfunctional, cause the most severe symptoms of scurvy. In animals, these reactions are especially important in wound-healing and in preventing bleeding from capillaries. Ascorbate may also act as an antioxidant against oxidative stress[8] . Designated chemically as 2-Oxo-L-threo-hexono-1,4-lactone-2,3-enediol.The molecular weight is 176.12 g/mol. Its molecular formula is C6H8O6 . The structural formula is:
Material
We used the Multi wall and Single wall carbon nanotube with 95%pure degree, production of neutrino Company. Vitamin C production of Pharmaceutical companies Arya pharmaceuticals
Apparatus
In this survey the spectrophotometer (Jenway6505, Model,England), magnetic stirrer (Heidolph, Mr3001 Model), Analytical balances (Sartorius Model), Filter paper (Albet), were used.
Adsorption experiments
Solutions used were prepared by solving Vitamin C distilled water is used twice. First asolution with concentration of 50 mgL-1 preparedthen it dilute and different concentrations (8,10,12,14ppm) has obtained. Certain quantities of carbonnano-tube (0.005 g) have been added as absorbent to 100 mL glasses containing 10 mL vitamin C solution, then it was stirred by mixer for 10 hours(optimal time).After mixing process then the solution should be put into 1000 rpm speed for 25 minutesuntil the solid and liquid phases are separated.Vitamin C concentration was measured before andalso after adsorption by atomic absorption machine.All tests have been performed at the lab with the temperature of(C° 2 ± 22).
Adsorption equilibrium isotherms
Equilibrium study on adsorption provides information on the capacity of the adsorbent. An adsorption isotherm is characterized by certain constant values, which express the surface properties and affinity of the adsorbent and can also be used to compare the adsorptive capacities of the adsorbent for different pollutants. Equilibrium data can be analyzed using commonly known adsorption systems. Several mathematical models can be used to describe experimental data of adsorption isotherms. The Freundlich, Langmuir and Temkin models are employed to analysis adsorption occurred in the experiment. Data of adsorption isotherms. The Freundlich, Langmuir and Temkin models are employed to analysis adsorption occurred in the experiment.
Langmuir model
The Langmuir adsorption model [9] is the most common model used to quantify the amount of adsorbs ate on an adsorbent as a function of partial pressure or concentration at a given temperature. This equation expressed by relation.
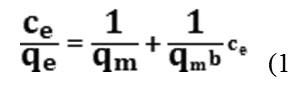
in this equation, qe (mg.g-1) is the solution was adsorbed the surface and qe is equilibrium constant of adsorption and b is the capacity of adsorption in saturated single layer and Ce the equilibrium concentration of the solute in the bulk solution (mg L-1).
Freundlich model
The Freundlich equation or Freundlich adsorption isotherm is an adsorption isotherm,which a curve is relating the concentration of a solute on the surface of an adsorbent, to the concentration of the solute in the liquid with which it is in contact. In 1909, Freundlich gave an empirical expression representing the isothermal variation of adsorption of a quantity of gas adsorbed by unit mass of solid adsorbent with pressure[10,11]. This equation is known as Freundlich adsorption Isotherm or Freundlich adsorption equation. This model is specified with equation.
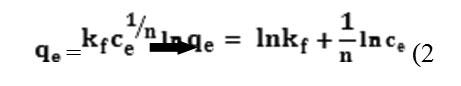
In this equation, qe (mg.g-1) is amount of absorbed material in absorbent surface, K; n in arrangement are adsorption capacity and adsorption intensification.
Temkin model:
The Temkin model is linearly represented as equation (3) and generally applied in the form:
qe = BlnA+BlnCe (3
Where A and B are the Temkin isotherm constant (L/g) and heat of sorption (J/mol)respectively[12,13]. R is the gas constant (J/mol/k), b is the Temkin isotherm constant linked to the energy parameter, B, as shown on equation:
b=RT/B (4
T is the absolute temperature in Kelvin.
Results and Discussion
Comparative The Langmuir, Freundlich and Temkin isotherms of the adsorption process of Vitamin C on MWCNT and SWCNTs are shown in figures 1 to 3 and calculated parameters of these models are shown in Table 1. It was observed that the experimental data were well represented by Langmuir, Freundlich and Temkin models.
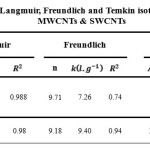 |
Table 1: Parameters of Langmuir, Freundlich and Temkin isotherms of the Vitamin C on MWCNTs & SWCNTs Click here to View table |
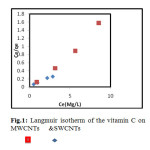 |
Fig.1: Langmuir isotherm of the vitamin C on MWCNTs & SWCNTs Click here to View figure |
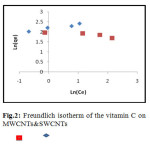 |
Fig.2: Freundlich isotherm of the vitamin C on MWCNTs & SWCNTs Click here to View figure |
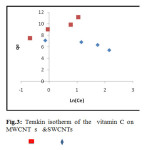 |
Fig.3: Temkin isotherm of the vitamin C on MWCNTs & SWCNTs Click here to View figure |
Conclusion
This study compares the adsorption isotherms of antioxidant drugs were studied. A principle of absorption spectroscopy is that the number of molecules absorbing certain wavelengths of light, the more is absorbed. 224 -240 nm range are absorbed antioxidant. We have studied the interaction of Vitamin C with as-prepared and purified multiwall (MWCNTs) and singlewall carbon nanotube (SWCNTs). Optical spectroscopic data Uv/Vis showed that purified carbon nanotubes interact with Vitamin C. in this research, adsorption of Antioxidant drug (Vitamin C) on multiwall and singlewall carbon nanotube from aqueous solution were studied. The results show that the amount of Vitamin C adsorbed on carbon nanotube surface increased with the increase of the Antioxidant concentration. The results show that correlation coefficient in langmuir isotherm model for multi-wall carbon nanotubewas higher and it’s efficiency in the removal of Vitamin C is better than single-wall carbon nanotube.The experimental results show that it was found that the Langmuir model described the adsorption process better than other two isotherm models.
References
- Wang, X.; Li, Qunqing; Xie, Jing; Jin, Zhong; Wang, Jinyon Li, Yan; Jiang, Kaili;Fan, Shoushan “Fabrication of Ultralong and Electrically Unifor Single-Walled Carbon Nanotubes on Clean Substrates. Nano Letters 9 (9):3137-3141, (2009).
- Single-Walled Carbon Nanotubes on Clean Substrates .Nanotechnology: A Guide to Nano- Objects.Chemical Engineering Progress 107 (5): 28–32.
- Flahaut, E.; Bacsa, Revathi; Peigney, Alain; Laurent, Christophe “Gram-Scale CCVD Synthesis of Double-Walled Carbon Nanotubes”.Chemical Communications 12 (12): 1442–1443, (2003)
- Cumings, J.; Zettl, A. “Low-Friction Nanoscale Linear Bearing Realized from Multiwall Carbon Nanotubes”. Science 289 (5479): 602–604, (2000)
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. “Exceptionally high Young’s modulus observed for individual carbon nanotubes”. Nature 381 (6584): 678–680, (1996)
- Zavalniuk, V.; Marchenko, S. “Theoretical analysis of telescopic oscillations in multi-walled carbon nanotubes”. Low Temperature Physics 37 (4). 337, (2011)
- Prabhat; Marcus Flather, Eva Lonn, Michael Farkouh, and Salim Yusuf (1995). The Antioxidant Vitamins and Cardiovascular Disease: A Critical Review of Epidemiologic and Clinical Trial Data. Annals of Internal Medicine 123 (11): 860–872.
- Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M (February 2003). “Vitamin C as an antioxidant: evaluation of its role in disease prevention”. J Am CollNutr22 (1): 18–35.
- J.Am, 1918,Langmuir, The adsorption of gases on pIane surfaces of glass, mica and platinum,.Chem.Soc. 1361-140
- H.M.F. Freundlich, U¨ ber die adsorption in lo¨sungen, Z. Phys. Chem. 57: 385-470 (1906). 38
- J. Zeldowitsch, Adsorption site energy distribution, ActaPhysicochim. URSS 1: 961- 973 (1934).
- M.I. Temkin, Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules, Zh. Fiz. Chim.15: 296– 332 (1941).
- Y.Kim,C.im,I.Choi,S.Rengraj,J.Yi,Environ. Sci. Technol. 38: 924 (2004)

This work is licensed under a Creative Commons Attribution 4.0 International License.









