Biodegradable Poly(D,L-Lactide)/Lipid Blend Microparticles Prepared by Oil-in-Water Emulsion Method for Controlled Release Drug Delivery
Yaowalak Srisuwan and Yodthong Baimark*
Biodegradable Polymers Research Unit, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahasarakham University, Mahasarakham 44150, Thailand
DOI : http://dx.doi.org/10.13005/ojc/300108
Article Received on : January 15, 2014
Article Accepted on : March 03, 2014
Article Published : 30 Mar 2014
The effects of blend ratio and drug loading content of poly(D,L-lactide) (PDLL)/stearic acid blends on microparticle characteristics and drug release behaviors were evaluated. The blend microparticles were prepared by an oil-in-water emulsion solvent evaporation method for drug delivery of a poorly water-soluble model drug, indomethacin. The microparticles were characterized using a combination of scanning electron microscopy (SEM), light scattering particle size analysis, differential scanning calorimetry (DSC) and UV-vis spectrophotometry. The blend microparticles with a PDLL/stearic acid blend ratio in the range 100/0-95/5 (w/w) exhibited a spherical shape with a smooth surface. Blend microparticles with a similar size (167-177 µm) and drug loading efficiency (60-67%) were obtained. The drug loading content did not affect the characteristics of the blend microparticles. An in vitro drug release test demonstrated that the level of drug release decreased as the stearic acid blend ratio increased and the drug loading content decreased. The overall results indicated that it was possible to use PDLL/stearic acid blend microparticles as a controlled release drug delivery system.
KEYWORDS:biodegradable polymer; poly(D;L-lactide); stearic acid; polymer blends; microparticles; drug delivery.
Download this article as:| Copy the following to cite this article: Srisuwan Y and Baimark* Y. Biodegradable Poly(D,L-Lactide)/Lipid Blend Microparticles Prepared by Oil-in-Water Emulsion Method for Controlled Release Drug Delivery. Orient J Chem 2014;30(1). |
| Copy the following to cite this URL: Srisuwan Y and Baimark* Y. Biodegradable Poly(D,L-Lactide)/Lipid Blend Microparticles Prepared by Oil-in-Water Emulsion Method for Controlled Release Drug Delivery. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2593 |
INTRODUCTION
Controlled release, prolonged release and sustained release are terms used to refer to drug delivery systems that are produced to deliver precise drug amounts at a pre-programmed rate to achieve the drug level necessary for treatment after administration of the system1,2. Controlled release drug delivery also attempts to maintain drug levels within the therapeutic level to reduce potentially hazardous peaks in drug concentration3. Biodegradable polymers have been widely used as a matrix for controlled release drug delivery systems in particles, film, fibre and gel forms. The removal of these biodegradable polymeric devices at the completion of therapy is not required. The drug release mechanisms of a biodegradable polymeric matrix consist of drug diffusion out and matrix erosion stages. Amorphous poly(D,L-lactide) (PDLL) is a synthetic biodegradable polymer. This induces uniform drug distribution in the PDLL matrix. PDLL microparticles have been widely investigated as controlled-release drug delivery systems4-7. The oil-in-water emulsion solvent evaporation technique has been usually used to prepare the PDLL microparticles containing poorly water-soluble model drugs4,7. Drug release from polyester particles depended upon the polyester type, polyester molecular weight, drug loading content, particle size and polymer blending8,9,10. Lipids are inexpensive and low toxicity substances that have been widely studied as a matrix for drug carriers11,12,13. Solid lipid microparticles attain high encapsulation efficiency for poorly water-soluble drugs due to their hydrophobic nature. Stearic acid microparticles combine the advantages of liposome and polymer microparticles, while avoiding some of their disadvantages such as toxicity, biodegradability problems and raw material costs14.However, PDLL/stearic acid blend microparticles for use as controlled release drug delivery applications have not been reported. In this paper, PDLL/stearic acid blend miroparticles containing a poorly water-soluble model drug were prepared by the oil-in-water emulsion solvent evaporation method. The influences of the PDLL/stearic acid blend ratio and drug loading content on morphology, particle size and thermal transition properties of the blend microparticles was determined. In vitro drug release behaviour was also investigated.
EXPERIMENTAL
Materials
Poly(D,L-lactide) (PDLL) was synthesized by ring-opening polymerization of a D,L-lactide monomer in bulk under a nitrogen atmosphere at 140°C for 24 h using 1-dodecanol (98%, Fluka) and stannous octoate (95% Sigma) as the initiator and catalyst, respectively. The number-average molecular weight and molecular weight distribution of the resulting PDLL obtained from the GPC method were 17,050 g/mol and 1.4, respectively. The 1-dodecanol was distilled under reduced pressure before use. Stannous octoate, stearic acid (95%, Sigma-Aldrich, USA), indomethacin model drug (99%, Sigma) and Tween80 (Labchem) were used without further purification. Dichloromethane (Carlo Erba) was used in analytical grade.
Preparation of blend microparticles
The PDLL/stearic acid blend microparticles containing the model drug were prepared by the oil-in-water emulsion solvent evaporation method. Briefly, 0.1 g of PDLL/stearic acid mixture was completely dissolved in 2.5 mL of dichloromethane before pouring into 400 mL of 2 wt% Tween80 aqueous solution under magnetic stirring to form an oil-in-water emulsion. The dichloromethane was evaporated in a fume hood for 6 h. The blend microparticles were collected by centrifugation and washed with distilled water three times. The obtained blend microparticles were then freeze-dried overnight and stored in a desiccator before characterization and in vitro drug release testing.
Characterization of blend microparticles
The morphology of the microparticles was determined by scanning electron microscopy (SEM) using a JEOL JSM-6460LV SEM. The microparticles were coated with gold to enhance conductivity before scanning. The average particle size of the microparticles was measured by light scattering (LS) analysis using a Coulter LS230 particle size analyzer at 25°C in a water medium. Thermal transition properties of the microparticles were carried out by means of differential scanning calorimetry (DSC) using a Perkin-Elmer DSC Pyris Diamond. For DSC analysis, 5–10 mg of sample was heated at 10oC/min under a helium flow. The drug loading content of the indomethacin model drug entrapped in the blend microparticles was determined by dissolving 20 mg of blend microparticles in 1 mL of dichloromethane. The indomethacin concentration in clear solution was determined from the absorbance at lmax = 319 nm by a UV-Vis spectrophotometry using a Perkin-Elmer Lambda 25 and compared to a standard curve of indomethacin. Theoretical drug loading content (DLCtheoretical), actual drug loading content (DLCactual) and drug loading efficiency (DLE) were calculated from equations (1)-(3), respectively. The DLCactual is an average value from three determinations.
Formula 1, 2, 3.
In vitro drug release test
An in vitro drug release test was performed in a phosphate buffer solution (PBS, 0.1 M, pH 7.4) at 37 °C under shaking. The blend microparticles (~10 mg) were placed in a dialysis tube before immersing in 200 mL of buffer. At predetermined time intervals, 10 mL of release medium was withdrawn. Then 10 mL of fresh buffer was added to the original to maintain the total volume. The drug release was determined by UV-vis spectrophotometry at lmax = 319 nm and compared to a standard curve of the drug solution. Cumulative drug release was calculated in terms of the ratio of the cumulative mass of the released drug at a given time against the initial drug loading in the blend microparticles. In vitro drug release tests were performed in triplicate (n = 3).
RESULTS AND DISCUSSION
Morphology and size of blend microparticles
The yield of the blend microparticles was in the range 85 – 92%. The morphology of the blend microparticles was determined from their SEM micrographs as illustrated in Fig. 1. All blend microparticles were observed to have fine dispersibility, and the microparticles did not aggregate. The results suggest that the preparation conditions, including polymer concentration, volumes of oil and water phases, stirring speed and evaporation time for the oil-in-water emulsion solvent evaporation method used in this work were appropriate for preparing the drug-loaded blend microparticles with a high yield and good dispersibility. Fig. 2 shows the
 |
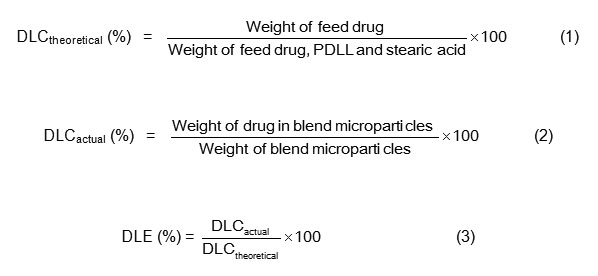
Fig. 1: SEM micrographs of the blend microparticles with PDLL/stearic acid blend ratios of (a) 100/0, (b) 97.5/2.5, (c) 95/5 and (d) 92.5/7.5 (w/w). All bars = 100 mm. Click here to View Figure |
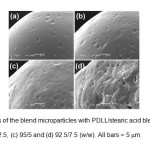 |
Fig. 2: Surfaces of the blend microparticles with PDLL/stearic acid blend ratios of (a) 100/0, (b) 97.5/2.5, (c) 95/5 and (d) 92.5/7.5 (w/w). All bars = 5 mm. Click here to View Figure |
RESULTS AND DISCUSSION
Morphology and size of blend microparticles
The yield of the blend microparticles was in the range 85 – 92%. The morphology of the blend microparticles was determined from their SEM micrographs as illustrated in Fig. 1. All blend microparticles were observed to have fine dispersibility, and the microparticles did not aggregate. The results suggest that the preparation conditions, including polymer concentration, volumes of oil and water phases, stirring speed and evaporation time for the oil-in-water emulsion solvent evaporation method used in this work were appropriate for preparing the drug-loaded blend microparticles with a high yield and good dispersibility. Fig. 2 shows the surfaces of the blend microparticles. The resulting blend microparticles with PDLL/stearic acid blend ratios of 100/0, 97.5/2.5 and 95/5 (w/w) were nearly spherical in shape with a smooth surface as shown in Figs. 1(a)-1(c) and 2(a)-2(c), respectively. This indicates that the PDLL/stearic acid blend ratio in this range did not affect the morphology of the microparticles. However, the 92.5/7.5 (w/w) PDLL/stearic acid blend microparticles were irregular in shape with a rough surface as shown in Figs. 1(d) and 2(d), respectively. This may be explained by the steraric acid molecules exhibiting self-aggregate to induce irregular microparticles and a rough surface for the 92.5/7.5 blend microparticles. The average particle size of the blend microparticles was determined by light scattering analysis. All of the particle size graphs exhibited similar unimodal particle size distributions, an example of which is shown in
 |
Fig. 3: Particle size graph for the 92.5/7.5 (w/w) PDLL/stearic acid blend microparticles. Click here to View Figure |
for the 92.5/7.5 (w/w) PDLL/stearic acid blend microparticles. The average particle size of the blend microparticles, as summarized in Table 1
Table 1: Particle size and drug loading of the blend microparticles.
|
PDLL/stearic acid (w/w) |
Average particle size (µm) |
DLCtheoretical (%) |
DLCactual (%) |
DLE (%) |
|
100/0 97.5/2.5 95/5 92.5/7.5 97.5/2.5 97.5/2.5 |
167 ± 62 175 ± 79 177 ± 53 167 ± 58 175 ± 64 168 ± 75 |
10 10 10 10 5 2.5 |
6.7 ± 1.5 6.4 ± 1.4 6.6 ± 1.5 6.2 ± 1.6 3.2 ± 1.1 1.5 ± 0.4 |
67 64 66 62 64 60 |
was in the range of 167 – 177 µm. The PDLL/stearic acid blend ratio did not affect the average particle size. surfaces of the blend microparticles. The resulting blend microparticles with PDLL/stearic acid blend ratios of 100/0, 97.5/2.5 and 95/5 (w/w) were nearly spherical in shape with a smooth surface as shown in Figs. 1(a)-1(c) and 2(a)-2(c), respectively. This indicates that the PDLL/stearic acid blend ratio in this range did not affect the morphology of the microparticles. However, the 92.5/7.5 (w/w) PDLL/stearic acid blend microparticles were irregular in shape with a rough surface as shown in Figs. 1(d) and 2(d), respectively. This may be explained by the steraric acid molecules exhibiting self-aggregate to induce irregular microparticles and a rough surface for the 92.5/7.5 blend microparticles. The average particle size of the blend microparticles was determined by light scattering analysis. All of the particle size graphs exhibited similar unimodal particle size distributions, an example of which is shown in Fig. 3 for the 92.5/7.5 (w/w) PDLL/stearic acid blend microparticles. The average particle size of the blend microparticles, as summarized in Table 1, was in the range of 167 – 177 µm. The PDLL/stearic acid blend ratio did not affect the average particle size.
Thermal properties of blend microparticles
The thermal transition properties of the blend microparticles were investigated by DSC. The DSC thermograms of the blend microparticles are compared in Fig. 4.
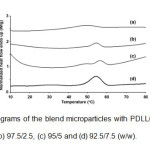 |
Fig. 4: DSC thermograms of the blend microparticles with PDLL/stearic acid blend ratios of (a) 100/0, (b) 97.5/2.5, (c) 95/5 and (d) 92.5/7.5 (w/w). Click here to View Figure |
The DSC results are summarized in Table 2.
Table 2: DSC results of the blend microparticles.
|
PDLL/stearic acid (w/w) |
DLCtheoretical (%) |
Tga (°C) |
Tmb (°C) |
DHmb (J/g) |
|
100/0 97.5/2.5 95/5 92.5/7.5 97.5/2.5 97.5/2.5 |
10 10 10 10 5 2.5 |
34 27 27 26 26 26 |
– 54 56 54 55 54 |
– 5.7 9.1 15.8 14.6 15.4 |
The DSC thermogram of PDLL microparticles in Fig. 4(a) showed a single glass transition temperature (Tg) at 34°C without a melting peak. The PDLL is amorphous. The blend microparticles exhibited a lower Tg (26 – 27°C) than the PDLL microparticles. This indicates that the PDLL and stearic acid were partial miscible. The blend microparticles also showed a single melting temperature (Tm) in the range 54 – 56°C due to stearic acid crystallization. The stearic acid showed a Tm at 54°C (data did not shown). In addition, the heat of melting (DHm) for the blend microparticles, which was directly related to stearic acid crystallinity, decreased as the stearic acid ratio increased, which supports the partial miscibility between the PDLL and stearic acid phases.
Drug loading and in vitro drug release of blend microparticles
The theoretical drug loading content (DLCtheoretical), actual drug loading content (DLCactual) and drug loading efficiency (DLE) calculated from equations (1) – (3), respectively are also summarized in Table 1. It was found that the PDLL microparticle sample showed the highest DLE (77%). All blend microparticles showed similar DLE values in the range 60 – 66%. This may be explained by the interactions of the stearic acid-indomethacin model drug that were weaker than the PDLL-indomethacin interactions during the microparticle formation. The DLCtheoretical value did not significantly affect the DLE value. The in vitro drug release of the indomethacin from the blend microparticles was determined in a phosphate buffer pH 7.4 at 37°C for 48 h. The influences of the PDLL/stearic acid blend ratio and DLCtheoretical value on the drug release behavior of the blend microparticles are illustrated in Figs. 5 and 6, respectively. The drug release profile is plotted between cumulative drug release and release time. An initial burst release effect within the first 6 h was observed followed with a further sustained release for all microparticles. The initial burst release occurred due to the immediate release of the drug on and near the microparticle surfaces. As shown in Fig. 5, the total
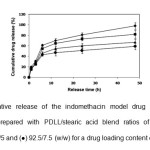 |
Fig. 5: Cumulative release of the indomethacin model drug from the blend microparticles prepared with PDLL/stearic acid blend ratios of (¨) 100/0, (■) 97.5/2.5, (▲) 95/5 and (●) 92.5/7.5 (w/w) for a drug loading content of 10%. Click here to View Figure |
cumulative drug release at 48 h decreased from 98% to 59% when the stearic acid ratio was increased from 0 to 7.5%. This may be due to the hydrophobicity of the stearic acid component. The total cumulative drug release at 48 h also decreased from 82% to 67% when the DLCtheoretical was decreased from 10% to 2.5% as shown in Fig. 6.
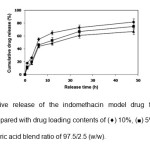 |
Fig. 6: Cumulative release of the indomethacin model drug from the blend microparticles prepared with drug loading contents of (¨) 10%, (■) 5% and (▲) 2.5% for the PDLL/stearic acid blend ratio of 97.5/2.5 (w/w). Click here to View Figure |
This may be explained by the high drug loading content that could affect the properties of the polymeric network in the microparticle matrix, thus affecting the diffusion barrier. The results of the drug release demonstrated that the drug release from the PDLL/stearic acid blend microparticles was controlled by the PDLL/stearic acid blend ratio and drug loading content.
CONCLUSION
The present work showed that drug-loaded PDLL/stearic acid blend microparticles with various blend ratios could be successfully prepared by the oil-in-water emulsion solvent evaporation method. The microparticles with PDLL/stearic acid blend ratios in the range of 100/0 – 95/5 (w/w) were spherical in shape and had smooth surfaces, but the 92.5/7.5 (w/w) blend microparticles were not. Particle size and drug loading efficiency of the PDLL and all the blend microparticles were similar. The thermal transition properties of the blend microparticles strongly depended upon the blend ratio. The release of indomethacin from the blend microparticles can be tailored by adjusting the PDLL/stearic acid blend ratio and drug loading content. These blend microparticles are considered to be promising drug carriers for controlled release of poorly water-soluble drugs.
ACKNOWLEDGEMENTS
This research was financially supported by Mahasarakham University, Mahasarakham, Thailand. Financial support from the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Office of the Higher Education Commission, Ministry of Education, Thailand is also gratefully acknowledged.
REFERENCES
- Mohammad, S., Fatemeh, S., Mojgan, Y. Controlled release of acetaminophen from CMC-based hydrogels. Orient. J. Chem. 27: 895-902 (2011).
- Kumar, V., Prajapati, S.K., Soni, G.C., Singh, M., Kumar, N. Sustained release matrix type drug delivery system: a review. World J. Pharm. Pharm. Sci. 1: 934-60 (2012).
- Brannon-Peppas, L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int. J. Pharm. 116: 1-9 (1995).
- Chung, T.W., Huang, Y.Y., Liu, Y.Z. Effects of the rate of solvent evaporation on the characteristics of drug loaded PLLA and PDLLA microspheres. Int. J. Pharm. 212: 161-169 (2001).
- Cui, F., Cun, D., Tao, A., Yang, M., Shi, K., Zhao, M. Preparation and characterization of melittin-loaded poly(DL-lactic acid) or poly(DL-lactic acid-co-glycolic acid) microspheres made by the double emulsion method. J. Controlled Release 107: 310-319 (2005).
- Liu, M., Zhou, Z., Wang, X., Xu, J., Yang, K., Cui, Q. Formation of poly(L,D-lactide) spheres with controlled size by direct dialysis. Polymer 48: 5767-5779 (2007).
- Baimark, Y., Srisa-ard, M. Preparation of drug-loaded microspheres of linear and star-shaped poly(D,L-lactide)s and their drug release behaviors. J. Appl. Polym. Sci. 124: 3871-3878 (2012).
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 21:2475-2490 (2000).
- Freiberg, S., Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 282: 1-18 (2004).
- Dong, Y., Feng, S.S. Nanoparticles of poly(D,L-lactide)/methoxy poly(ethylene glycol)-poly(D,L-lactide) blends for controlled release of paclitaxel. J. Biomed. Mater. Res. 78A: 12-19 (2006).
- Muller, R.H., Mader, K., Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Euro. J. Pharm. Biopharm. 50: 161-177 (2000).
- Jaspart, S., Bertholet, P., Piel, G., Dogne, J.M., Delattre, L., Evrard, B. Solid lipid microparticles as a sustained release system for pulmonary drug delivery. Euro. J. Pharm. Biopharm. 65: 47-56 (2007).
- Perge, L., Robitzer, M., Guillemot, C., Devoisselle, J.M., Quignard, F., Legrand, P. New solid lipid microparticles for controlled ibuprofen release: formulation and characterization study. Int. J. Pharm. 422: 59-67 (2012).
- Dalpiaz, A., Mezzena, M., Scatturin, A., Scalia, S. Solid lipid microparticles for the stability enhamcement of the polar drug cyclopentyladenosine. Int. J. Pharm. 355:81-86 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









