The One Pot Synthesis Salicylaldehyde Prepared by Reactive Grinding with Derivative Phenol and Paraformaldehyde in Absence Magnesium Methoxide
Ebrahim Balali1, Abolghasem Shameli2*, Hoseein Naeimi3, Mohammad Mehdi Ghanbari4
1Department of Chemistry, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran 2Department of Chemistry, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran 3Organic Chemistry of Department, Faculty of Chemistery, Kashan University, Kashan, Iran 4Department of Chemistry, Sarvestan Branch, Islamic Azad University, Sarvestan, Iran
DOI : http://dx.doi.org/10.13005/ojc/290445
Article Received on :
Article Accepted on :
Article Published : 07 Dec 2013
The formylation phenols are mono-formylated using a mixture of paraformaldehyde, Mg(OMe)2, by reactive grinding. In all cases but one, only one regioisomer of the salicylaldehyde is obtained in good to high yield.
KEYWORDS:formylation;phenols;regioisomer;salicylaldehyde
Download this article as:| Copy the following to cite this article: Balali E, Shameli A, Naeimi H, Ghanbari M. M. The One Pot Synthesis Salicylaldehyde Prepared by Reactive Grinding with Derivative Phenol and Paraformaldehyde in Absence Magnesium Methoxide. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Balali E, Shameli A, Naeimi H, Ghanbari M. M. The One Pot Synthesis Salicylaldehyde Prepared by Reactive Grinding with Derivative Phenol and Paraformaldehyde in Absence Magnesium Methoxide. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1121 |
Introduction:
The formylation of the hydroxy group is one of the most widely used transformation in organic synthesis. Several synthetic methods for the formylation of primary and secondary Phenol to salicylaldehyde are known. Formylation of aromatic compounds is an important reaction in synthetic organic chemistry, and numerous methods are available.[1] By directed ortho-metallation of an activated phenol, a formyl group can be introduced selectively,[2] but this methodology requires the introduction and removal of the activating group for the synthesis of salicylaldehydes. The regioselectivity is even more of a problem for 1,3-dihydroxylated phenols (resorcinols). The recently reported regioselective ortho-formylation of substituted phenols using the MgCl2–Et3N base system and paraformaldehyde affords salicylaldehydes in excellent yields.[3] The salicylaldehydes obtained by this method have been employed by us and others for the preparation of useful products and intermediates.[4] We wanted to extend this methodology in absence Mg(OMe)2 to substituted mono-protected Phenols, a structural feature found in many natural products and biologically active substances(scheme 1).[5]
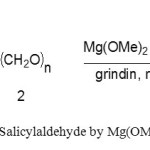 |
Scheme1. Synthesis Salicylaldehyde by Mg(OMe)2 in under grinding Click here to View figure |
Experimental
Melting points were measured on an Electrothermal 9100 apparatus. Elemental analyses for C, H, and N were performed using a Heraeus CHNO-Rapid analyzer. IR spectra were measured on a Shimadzu IR-460 spectrometer.1H and 13C NMR spectra were measured with a Bruker DRX-300 Avance instrument with CDCl3 as solvent at 300 and 75 MHz, respectively. Mass spectra were recorded on a Finnigan-Matt 8430 mass spectrometer operating at an ionization potential of 70 eV. Phenols and Paraformaldehyde, were obtained from Fluka and were used without further purification. Magnesium Methoxide was prepared by known methods.
A aromatic Phenol 1 (0.1 mol), magnesium methoxide (5 gr ), and the mixture was grinding to 1 minute at room temperature. A slurry of paraformaldehyde powder[6] (4 gr) in under grinding was added in small portions over 2 min to the reaction mixture. Stirring was continued at r.t for 8 min, after which added slowly to 10% sulfuric acid (20 ml g). The resulting mixture was stirred for 10 min, after which the aqueous layer was separated and extracted with ethyl acetate (2 x 100 ml). The combined organic layers and extracts were washed with 10% sulfuric acid (20 ml) and water (20 ml) and evaporated under reduced pressure to give the salicyaldehyde 3.
Salicylaldehyde, mp:430C, IR(KBr, cm-1): 2760, 1740. 1H NMR (300 MHz, CDCl3, ppm) δ: 7/75(m,4H), 9/9(S, 1H), 11/1 (S,1H), 13C NMR (75 MHz, CDCl3, ppm) δ: 117.45, 119.74, 120.53, 133.64, 136.88 , 161.45, 196.53. [M]+. found 122.040; calc.122.098.
2-hydroxy-5-methylbenzaldehyd, mp: 560C, IR(KBr, cm-1): IR:2770(W), 1740(C=O) 1H NMR (300 MHz, CDCl3, ppm) δ: 2/1(S,3H),6/2-6/6 (d, 2H),7(s,1H) , 9/4 (S, 1H), 10/7 (S,1H), 13C NMR (75 MHz, CDCl3, ppm) δ: 18.3 , 116.5, 119 , 122, 137.5, 142.5, 163.5, 195.5, [M]+. found 136.05; calc.136.07.
2-hydroxy-3-methylbenzaldehyd, mp: 580C, IR(KBr, cm-1): IR:2770, 1740 1H NMR (300 MHz, CDCl3, ppm) δ: 1/95(S,3H),6/2-6/6 (d, 2H),6/8(s,1H) , 9/3 (S, 1H), 10/9 (S,1H).
2-hydroxy-4-methylbenzaldehyd, mp: 60.50C, IR(KBr, cm-1): 2780, 1660, 1H NMR (300 MHz, CDCl3, ppm) δ: 1/95(S,3H),6/2-6/4 (d, 2H),7(s,1H) , 9/3 (S, 1H), 10/5 (S,1H), 13C NMR (75 MHz, CDCl3, ppm) δ: 22.4, 118, 119, 121.5, 134, 149 (4-C), 162, 196.
2-hydroxy-5-Boromobenzaldehyd mp: 750C, IR(KBr, cm-1): 2780, 1670, 1H NMR (300 MHz, CDCl3, ppm) δ: 6/4-6/9 (d, 2H),7/5(s,1H) , 9/7 (S, 1H), 10/7 (S,1H).
2-hydroxy-3,5-dimethyl benzaldehyd, mp: 620C, IR(KBr, cm-1): 2760, 1660, 1H NMR (300 MHz, CDCl3, ppm) δ: 1/8(S,6H),6/3-6/4 (m, 2H), 9/3 (S, 1H), 10/7 (S,1H)
Result and Discussion
The production in high yield (Table 1) of Salicylaldhyde derivatives (2) by means of two successive ortho-regioselective reactions on Phenol of the distribution of the products. In order to control the complexity of this reaction and direct it towards synthetic utility, we studied the reactions of formaldehyde with magnesium phenoxides which, as shown by Robert Aldred and co tend to react regioselectively with several reagents.[7]
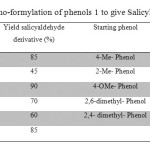 |
Table 1: ortho-formylation of phenols 1 to give Salicylaldoximes 3 Click here to View table |
para-Electron withdrawing substituent’s on the starting material phenols (Table 1) give rise to reduced reactivity and poorer formylation yield whilst the converse is true for electron-donating substituent’s. Bulky substituent’s in the Ortho position give rise to increased formation of by-products, presumably, to steric hindrance inhibiting coordination of formaldehyde to magnesium (necessary for formylation) resulting in increased base catalysed (but not magnesium mediated) by-product generation. Bulky substituent’s meta to the starting material phenol hydroxyl direct formylation to the position ortho to the hydroxyl and opposite the meta substituent. Smaller meta substituents direct formylation to the less hindered position Ortho to oxygen, but give rise to a mixture of products formylated in either the 2 or the 6 positions. Substituent’s in the Ortho position able to coordinate to the magnesium cation result in partial or complete inhibition of formylation, presumably due to chelation of magnesium which precludes the coordination of formaldehyde necessary for formylation.
Conclusion
we have extended our simple and regioselective ortho-formylation protocol to mono-protected substituted phenols. The formylations occurred with high to excellent yields.
Acknowledgment
Financial support to Pharmaceutical Sciences Branch Islamic Azad University of Tehran is gratefully acknowledged.
References
- Olah, G. A.; Ohannasian, L.; Arvanaghi, M. Chem. Rev. 1987, 87, 671.
- (a) Gilman, H.; Bebb, R. L. J. Am. Chem. Soc. 1939, 61, 109; (b) Wittig, G.; Fuhrman, G. Chem. Ber. 1940, 73, 1197; (c) Hartung, C. G.; Snieckus, V. In Modern Arene Chemistry; Astruc, D., Ed.; Wiley-VCH: Weinheim, 2002; Vol. 1, pp 330–363.
- Akselsen, Ø. W.; Skattebøl, L.; Hansen, T.V. Tetrahedron Lett. 2009, 50, 6339–6341
- a ) Anwar, H. F.; Hansen, T. V. Tetrahedron Lett. 2008, 49, 4443; (b) Anwar, H. F.; Skattebøl, L.; Hansen, T. V. Tetrahedron 2007, 63, 9997; (c) Hansen, T. V.; Skattebøl, L. Tetrahedron Lett. 2005, 46, 3829; (d) Hansen, T. V.; Skattebøl, L. Tetrahedron Lett. 2005, 46, 3357; (e) Odlo, K.; Klaveness, J.; Rongved, P.; Hansen, T. V. Tetrahedron Lett. 2006, 47, 1101; (f) Anwar, H. F.; Skattebøl, L.; Skramstad, J.; Hansen, T. V. Tetrahedron Lett. 2005, 46, 5285; (g) Wright, B. J. D.; Hartung, J.; Peng, F.; Van de Water, R.; Liu, H.; Tan, Q.-H.; Chou, T.-C.; Danishefsky, S. J. J. Am. Chem. Soc. 2008, 130, 16786; (h) Byun, J. H.; Kim, H. Y.; Kim, Y. S.; Mook- Jung, I.; Kim, D. J.; Lee, W. K.; Yoo, K. H. Bioorg. Med. Chem. Lett. 2008, 18, 5591; (i) Nichols, D. E.; Frescas, S. P.; Chemel, B. R.; Rehder, K. S.; Zhong, D.; Lewin, A. H. Bioorg. Med. Chem. 2008, 16, 6116; (j) Chen, Y.; Steinmetz, M. G. Org. Lett. 2005, 7, 3729; (k) Ueno, T.; Koshiyama, T.; Ohashi, M.; Kondo, K.; Kono, M.; Suzuki, A.; Yamane, T.; Watanabe, Y. J. Am. Chem. Soc. 2005, 127, 6556.
- S. M. Vahadat and M. Akbari., Orient. J. Chem., 27(4), 1573-1580 (2011)
- (a) Winssinger, N.; Barluenga, S. Chem. Commun. 2007, 22; (b) Kozubek, A.; Tyman, J. H. P. Chem. Rev. 1999, 99, 1; (c) Pandey, J.; Jha, A. K.; Hajela, K. Bioorg. Med. Chem. 2004, 12, 2239; (d) Shi, Y.-L.; Shi, M. Org. Biomol. Chem. 2007, 5, 1499; (e) Anwar, H. F.; Hansen, T. V. Org. Lett. 2009, 11, 587; (f) Kamat, V. S.; Chuo, F. Y.; Kubo, I.; Nakanishi, K. Heterocycles 1981, 15, 1163; (g) Shinonaga, H.; Kawamura, Y.; Ikeda, A.; Aoki, M.; Sakai, N.; Fujimoto, N.; Kawashima, N. Tetrahedron 2009, 65, 3446.; (h) Balalaie, S.; Azizian, J.; Shameli, A.; Bijanzadeh, H.R. Synthetic Commun. 2013, 43, 1787–1795.; (i) Azizian, J.; Shameli, A.; Balalaie, S.; Ghanbari, M. M.; Zomorodbakhsh, S.; Entezari, M.; Bagheri, S.; Fakhrpour, G. Orient. J. Chem., 2012, 28(1), 327-332
- Aldred, R.; Johnston, R.; Levin, D.; Neilan, J. J. Chem. Soc. Perkin Trans. 1 1994, 1823b) Casiraghi, G.; Casnati, G.; Cornia, M.; Pochini, A.; Puglia, G.; Sartori, G.; Ungaro, R. J. Chem. SOC.Perkin Trans. I , 1978,318. c) Casiraghi, G.; Casnati, G.; Puglia, G.; Sartori G.; Terenghi, G. J. Chem. Soc. Perkin Trans. I, 1980,862.

This work is licensed under a Creative Commons Attribution 4.0 International License.









