Synthesis Of Flavone Skeleton By Different Methods
R.B.Kshatriya Y. I. Shaikh, G.M.Nazeruddin*
Department of Chemistry (P.G. & Research Centre), Poona College of Arts, Science & Commerce, Pune, India.
DOI : http://dx.doi.org/10.13005/ojc/290425
Article Received on :
Article Accepted on :
Article Published : 16 Jan 2014
Flavones (flavus = yellow), are a class of flavonoids based on the backbone of 2-phenylchromen-4-one. Flavones are mainly found in cereals and herbs. Flavones are biologically active compounds. Therefore number of synthetic methods were developed. In this mini revive we have tried to cover various synthetic strategies for the synthesis of flavones. Some of the well known methods used for synthesis of flavones are Baker & Venkatraman synthesis and Claisen-Schmidt condensation.
KEYWORDS:Flavones; Biologically Active Compounds; Synthetic Methods
Download this article as:| Copy the following to cite this article: Kshatriya R.B, Shaikh Y. I, Nazeruddin G.M. Synthesis Of Flavone Skeleton By Different Methods. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Kshatriya R.B, Shaikh Y. I, Nazeruddin G.M. Synthesis Of Flavone Skeleton By Different Methods. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1769 |
Introduction
Flavones (flavus = yellow), are a class of flavonoids based on the backbone of 2-phenylchromen-4-one Apart from flavones other flavonoids are isoflavonoids, derived from 3-phenylchromen-4-one structure neoflavonoids, derived from 4-phenylcoumarine structure.The three flavonoid classes are all ketone-containing compounds, and as such, are anthoxanthins (flavones and flavonols)
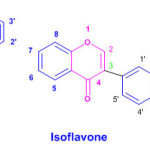 |
Click here to View figure |
Flavones are well known for their various biological activities such as anticancer1 Anti inflammatory2, anti-osteoporotic3, anti-diabetic4, etc. some of the examples as shown as under,
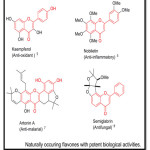 |
Table 1: Click here to View figure |
Synthetic strategies of flaovones
Traditionally, flavones have been prepared by BakerVenkatramanrearrangement
and Claisen-Schmidt condensation.which involves the conversion of 2hydroxyacetophenones into benzoyl esters, followed by rearrangement in base to1,3diphenylpropane1,3diones which upon cyclization under acidic conditions furnishes flavones.On the other hand hydroxychalcone synthesized from 2hydroxyacetophenone anbenzaldehyde under ClaisenSchmidt conditions can undergo oxidative cyclization to furnish flavones ring.
 |
Click here to View figure |
Basic schemes related to synthesis of flavones is mentioned below (Scheme 1-43)
 |
Scheme1 Click here to View figure |
Solvent free synthesis of flavone is carried out by Julia & co-workers10
Scheme2
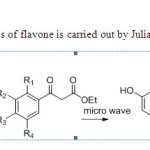 |
Schem2 Click here to View figure |
Flavones via a Microwave-Assisted, One-Pot Sonogashira−Carbonylation−Annulation Reaction is used by E.Awuah & A.Capretta11.
Scheme 3
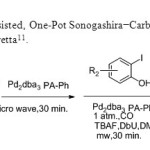 |
Scheme3 Click here to View figure |
Scheme 4: Photo cyclization of 2-Chloro-Substituted 1,3-Diarylpropan-1,3-diones to Flavones is invented by B.Kosmrrji & co-workers12.
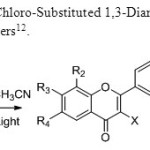 |
Scheme 4 Click here to View figure |
Scheme 5.Coversion of intermediate 1,3 dione is carried by G.Romanelli & co-workers13.
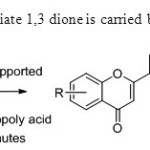 |
Scheme5.Coversion of intermediate 1,3 dione is carried by G.Romanelli & co-workers13. Click here to View figure |
Scheme 6. Alkene hydrogen is replaced by L.Klier & T.Bresser14.
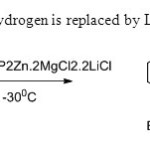 |
Scheme 6. Alkene hydrogen is replaced by L.Klier & T.Bresser14. Click here to View figure |
Scheme 7: A Novel Synthesis of 4H-Chromen-4-ones via Intramolecular Wittig Reaction is used for the synthesis of flavones15.
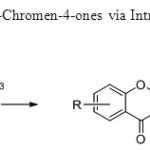 |
A Novel Synthesis of 4H-Chromen-4-ones via Intramolecular Wittig Reaction is used for the synthesis of flavones15. Click here to View figure |
Scheme8.This invention converts 1,3 dione into flavones.Only base is used for this purpose16.
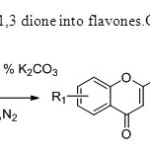 |
Scheme8.This invention converts 1,3 dione into flavones.Only base is used for this purpose16. Click here to View figure |
Scheme9.Koneni & his group first time invented flavones in which oxygen of flavone come from watr molecule17.
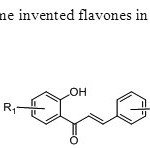 |
Scheme9.Koneni & his group first time invented flavones in which oxygen of flavone come from watr molecule17. Click here to View figure |
Scheme10. A two step synthesis of flavones via Wacker oxidation is carried out in this process18.
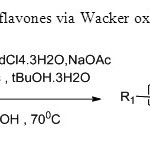 |
Scheme10. A two step synthesis of flavones via Wacker oxidation is carried out in this process18. Click here to View figure |
Scheme11. G.Kabalka & A.Meredy carried microwave assisted synthesis of flavones.Copper chloride is used as a catalyst for this process19.
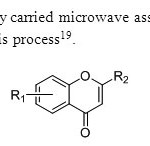 |
Scheme11. G.Kabalka & A.Meredy carried microwave assisted synthesis of flavones.Copper chloride is used as a catalyst for this process19. Click here to View figure |
Scheme12. Photo-Wittig reaction is apllied for the synthesis of flavones20.
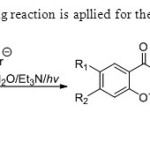 |
Scheme12. Photo-Wittig reaction is apllied for the synthesis of flavones20. Click here to View figure |
Scheme13. Oxidative cyclisation of chalcone to flavone is carried out for the synthesis of flavones.Here n-tetrabutylammonium tribromide is used as a catalyst21.
 |
Scheme13. Oxidative cyclisation of chalcone to flavone is carried out for the synthesis of flavones.Here n-tetrabutylammonium tribromide is used as a catalyst21. Click here to View figure |
Scheme14. 2’allyoxy chalcone undergoes oxidative coupling when treated with iodine & DMSO22.
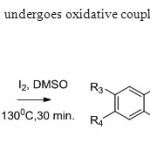 |
Scheme14. 2’allyoxy chalcone undergoes oxidative coupling when treated with iodine & DMSO22. Click here to View figure |
Scheme15.Palladium acetate is used catalyst for the synthesis of flavones23.
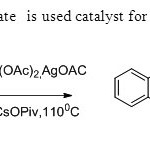 |
Scheme15.Palladium acetate is used catalyst for the synthesis of flavones23. Click here to View figure |
Scheme16. Construction of flavones through regioselective carbonylative annulation of 2 bromo phenols & terminal alkynes is carried out24.
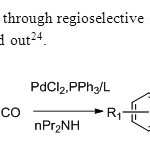 |
Scheme16. Construction of flavones through regioselective carbonylative annulation of 2 bromo phenols & terminal alkynes is carried out24. Click here to View figure |
Scheme17. Ganguly’s synthesis includes synthesis of flavones using O-hydroxy acetophenone & acetyl chloride as a precursor25.
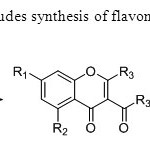 |
Scheme17. Ganguly’s synthesis includes synthesis of flavones using O-hydroxy acetophenone & acetyl chloride as a precursor25. Click here to View figure |
Scheme18. One pot synthesis of flavones using ferric chloride is efficient method carrid out by Rajiv Karmarkar & co-worker26.
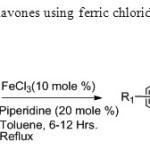 |
Scheme18. One pot synthesis of flavones using ferric chloride is efficient method carrid out by Rajiv Karmarkar & co-worker26. Click here to View figure |
Scheme19. Silica supported lewis acids indium chloride & indium bromide undergoes oxidative coupling to give flavones27.
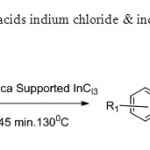 |
Scheme19. Silica supported lewis acids indium chloride & indium bromide undergoes oxidative coupling to give flavones27. Click here to View figure |
Scheme20.Wet acetone is efficient catalyst for the one pot synthesis of flavones from 2-hydroxy acetophenone & acetyl chloride28.
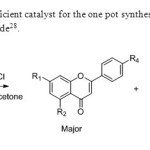 |
Scheme20.Wet acetone is efficient catalyst for the one pot synthesis of flavones from 2-hydroxy acetophenone & acetyl chloride28. Click here to View figure |
Scheme21. Formation of 1,3 dione using LiHDMs followed by cyclisation using acid catalyst is achived29.
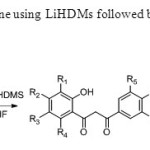 |
Scheme21. Formation of 1,3 dione using LiHDMs followed by cyclisation using acid catalyst is achived29. Click here to View figure |
Scheme 22. Carbonylative couplig using Pd catalyst is invention of this method30
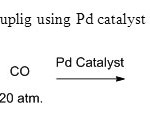 |
Scheme 22. Carbonylative couplig using Pd catalyst is invention of this method30 Click here to View figure |
Scheme 23. Daniel etal31suggested the following methodology consisting of five steps.
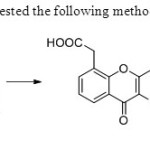 |
Scheme 23. Daniel etal31suggested the following methodology consisting of five steps. |
Scheme 24. Iodo & bromo derivatives of flavones were synthesized by this method32.
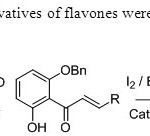 |
Scheme 24. Iodo & bromo derivatives of flavones were synthesized by this method32. Click here to View figure |
Scheme 25. Oxidative cyclisation followed by bromination is carried out by this process33.
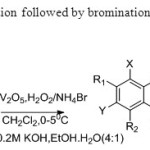 |
Scheme 25. Oxidative cyclisation followed by bromination is carried out by this process33. Click here to View figure |
Scheme 26.Base is used for cyclisation of inermediate to flavone34.
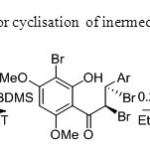 |
Scheme 26.Base is used for cyclisation of inermediate to flavone34. Click here to View figure |
Scheme 27. Wittig reaction is applied for the synthesis of flavones35.
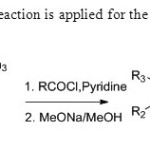 |
Scheme 27. Wittig reaction is applied for the synthesis of flavones35. Click here to View figure |
Scheme 28. Frédéric etal36suggested
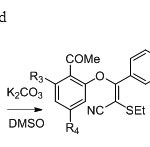 |
Scheme 28. Frédéric etal36suggested Click here to View figure |
Scheme 29 30. Dhanapalan N37 etal and Scheme30. Yoshida etal38 suggested the following methodologies respectively
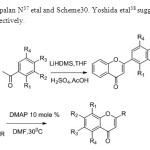 |
Scheme 29 30 Click here to View figure |
Scheme31.Hydrogen peroxide is used as catalyst for this one pot method39.
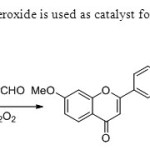 |
Scheme31.Hydrogen peroxide is used as catalyst for this one pot method39. Click here to View figure |
Scheme 32.Lewis acid ferric chloride is capplied for the synthesis of flavones via oxidative coupling by Kumar & Perumal40.
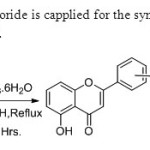 |
Scheme 32.Lewis acid ferric chloride is capplied for the synthesis of flavones via oxidative coupling by Kumar & Perumal40. Click here to View figure |
Scheme 33 Zanwar,M. R41suggested the following methodology.
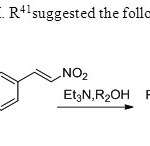 |
Scheme 33 Zanwar,M. R41suggested the following methodology. Click here to View figure |
Scheme 34.Yitterbium triflate is ude for the one pot synthesis of flavones in this paper42.
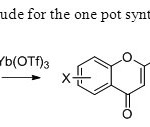 |
Scheme 34.Yitterbium triflate is ude for the one pot synthesis of flavones in this paper42. Click here to View figure |
Scheme 35.Suzuki-Miyaura coupling used for the synthesis of flavone by Kraus & Gupta43.
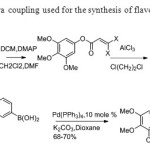 |
Scheme 35.Suzuki-Miyaura coupling used for the synthesis of flavone by Kraus & Gupta43. Click here to View figure |
Scheme 36.Zambre& Sangshetti used oxalic acid for oxidative coupling method44.
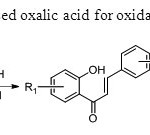 |
Scheme 36.Zambre& Sangshetti used oxalic acid for oxidative coupling method44. Click here to View figure |
Scheme 37. Iodine is used as catalyst for both Clause-Schmit condensation and oxidative coupling45.
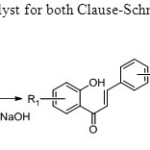 |
Scheme 37. Iodine is used as catalyst for both Clause-Schmit condensation and oxidative coupling45. Click here to View figure |
Scheme 38. Bosale & Sarda used ionic liquid for the synthesis of flavones from dione intermediate46.
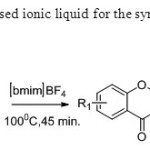 |
Scheme 38. Bosale & Sarda used ionic liquid for the synthesis of flavones from dione intermediate46. Click here to View figure |
Scheme 39.Jae In Lee etal47suggested the following methodology.
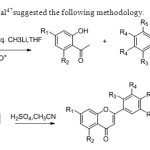 |
Scheme 39.Jae In Lee etal47suggested the following methodology. Click here to View figure |
Scheme 40. Sodium tellurim oxide is used for the oxidativ coupling method by the author KumarS& Sharma D. 48
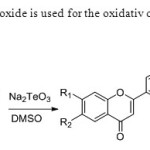 |
Scheme 40. Sodium tellurim oxide is used for the oxidativ coupling method by the author KumarS& Sharma D. 48 Click here to View figure |
Scheme 41.New catalyst at present is use of hetro polyacid is used for the synthesis of flavones.This solvent free synthesis avoids excess loss of solvents. 49
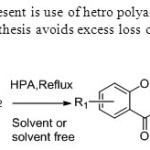 |
Scheme 41.New catalyst at present is use of hetro polyacid is used for the synthesis of flavones.This solvent free synthesis avoids excess loss of solvents. 49 Click here to View figure |
Scheme 42. CuI is another important catalyst invented by Zhiyun, Du & Huifen N..This method gives new catalyst for oxidative coupling of flavones50.
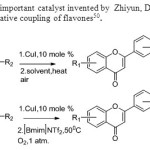 |
Scheme 42. CuI is another important catalyst invented by Zhiyun, Du & Huifen N..This method gives new catalyst for oxidative coupling of flavones50. Click here to View figure |
Scheme 43.Ortho acetyl acetophenone get converted to flavone directly without conversion to 1,3 dione intermediate51.
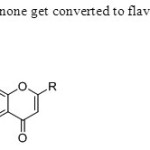 |
Scheme 43.Ortho acetyl acetophenone get converted to flavone directly without conversion to 1,3 dione intermediate51. Click here to View figure |
Conclusion
In conclusion I try to give most of the schemes related to flavones. This review provides ready data for researchers working in this field and skeleton flavone can be taileored to various novel Pharmacophore asper the need.
References:
- Liu, H. L.; Jiang, W. B.; Xie, M. X. Recent Pat. Anti-Cancer Drug Discovery 2010, 5, 152-164.
- Manthey, J. A.; Grohmann, K.; Montanari, A.; Ash, K.; Manthey, C. L. J. Nat Prod. 1999, 62, 441-444.
- Maurya, R.; Rawat, P.; Sharan, K.; Siddiqui, J. A.; Swarnkar, G.; Mishra, G.; Manickavasagam, K.; Arya, R.; Chattopadhyay, N. World Pat. 110003, 2009.
- Kunimasa, K.; Kuranuki, S.; Matsuura, N.; Iwasaki, N.; Ikeda, M.; Ito, A.; Sashida, Y.; Mimaki, Y.; Yano, M.; Sato, M.; Igarashi, Y.; Oikawa, T. Bioorg Med. Chem. Lett. 2009, 19, 2062-2064.
- Gabrielska, J.; Soczyńska-Kordala, M.; Przestalski, S. J. Agric. Food Chem. 2005, 53, 76-83.
- Martens, S.; Mithofer, A. Phytochemistry 2005, 66, 2399-2407.
- Halliwell, B.; Aeshbach, R.; Loliger, J.; Aruoma, O. I.; Food Chem. Toxicol. 1995, 33, 601-617.
- Ammar, N. M.; El-Diwany, A. I. J. Islamic Acad. Sci. 1988, 1, 72-73.
- Hua M.; Zhen Y. Org. Lett., 2000, 2 (12), 1765–1768.
- Julio A. S.; Pilar V.T.; Raquel C.R., J. Org. Chem., 2005, 70 (7),2855–2858
- Emelia A. ; Alfredo C., Org. Lett., 2009, 11 (15), 3210–3213
- Berta K.;Boris Š. Org. Lett., 2007, 9 (20), 3993–3996
- Gustavo P. R.; Virla,E.G.; Duchowicz,P.R. Ana, L. G.; Diego M. R.; Daniel O. B., Erlinda del V. O. ; Autino, J.C. J. Agric. Food Chem., 2010, 58 (10),6290–6295.
- Lydia, K.; Tomke, B.; Tobias, A.; Nigst, K.; Knochel,K J. Am. Chem. Soc., 2012, 134 (33), 13584–13587
- Kumar,K.; Bodas M. S. Org. Lett., 2000, 2 (24),3821–3823
- Jie, Z.; Yufen,Z.; Hua, F. Org. Lett., 2012, 14 (11), 2710–2713.
- Sashidhara,K.V. ;Kumar,M.; Kumar,A. Tetrahedron Letters, 53(18) 2012,2355-2359.
- Michael, L.; Kabir,M.S.; Cook,J.M. Tetrahedron Letters, 51(7) ,17, 2010, 1095-1098.
- Kabalka,G.W. Mereddy,A.R. Tetrahedron Letters, 46(37) , 2005, 6315-6317.
- Das,J.; Ghosh,S. Tetrahedron Letters, 52(52), 2011, 7189-7194.
- Bose,G.; Mondal,E.; Khan,A.T. ; Bordoloi,M. J. Tetrahedron Letters, 2001, 42,(50), 8907-8909.
- Lokhande,P.D.; Sakate,S.S.;Taksande, K. N.; Navghare,B.Tetrahedron Letters, 46, (9), 2005, 1573-1574.
- Kim,K.H.; Lee,H.S.; Kim,S.H. ;Kim,J.S. Tetrahedron Letters, 2012(53)22, 2761-2764.
- Jianming, L.; Muwen, L.;Yuanyuan ,Y.; Ningfei, Z.; Yuanli, Z.; Kelei, Z. Tetrahedron Letters,2013,54(14), 1802-1807.
- Ganguly,A.K. ; Kaur,S.; Mahata,P.K. ; Biswas,D.; Pramanik,B.N. ; Chan,T.M. Tetrahedron Letters,2005,46(23), 4119-4121.
- Maiti,M.; Karmakar,R.; Bhattacharya,R.N.; Kayal,K. Tetrahedron Letters, 52(43) 2011,5610-5612.
- Naseem Ahmed, Hasrat Ali, Johan E. van Lier Tetrahedron Letters, 46(2), 2005, 253-256
- Chin Fei Chee, Michael J.C. Buckle, Noorsaadah Abd. Rahman Tetrahedron Letters,52(24), 2011, 3120-3123.
- Mark Cushman, Dhanapalan Nagarathnam Tetrahedron Letters 1990, 31,6497-6500
- V.N. Kalinin, M.V. Shostakovsky, A.B. Ponomaryov Tetrahedron Letters,1990 , 31, 4073-4076.
- Daniel ,D.; Laetitia, M. Tetrahedron Letters,1995,36,1845-1848.
- Pinto,D.; Silva,A.S.; Cavaleiro,J.S. TetrahedronLetters, 1994, 35, 9459-9460
- Khan,A.T.; Goswami,P. Tetrahedron Letters,2005 46, 4937-4940
- Abu T. Khan, Abhik Choudhury, Shahzad Ali, Md. Musawwer Khan Tetrahedron Letters, 2012,53,4852-4857.
- Yves Le, F.;Martine L. Tetrahedron Letters,1986,27,5503-5504.
- Frédéric Lassagne, Francis Pochat Tetrahedron Letters, Volume 2003,44, 22 ,9283-9285.
- Dhanapalan N., Mark C. Tetrahedron 1991 , 47, 5071-5076.
- Yoshida, Y.F.; Koya S.; Takayuki,D.Tetrahedron, 67, 23 , 2011, 9993-9997 .
- Francesco F.; Giosanna P.; Oriana P.; Ferdinando P. Tetrahedron 1994,50, 39, 11499-11508.
- Kumar,K.H.; Perumal,P.T. Tetrahedron,2007,63,38, 9531-9535
- Zanwar,M. R.; Raihan,M. J.; Gawande,S.D; Kavala,V; Janreddy, V.;Chun-Wei K.; Ambre,R. ; Ching-Fa,Y. J. Org. Chem., 2012, 77,495–6504.
- Eric F.; Dumas,A. M.; Kuropatwa, B. A.; Malhotra,N.R.; Sitler, T. C. J. Org. Chem., 2006, 71, 409–412
- Kraus, G.A.; Gupta, V. Org. Lett. 2010, 12, 5278–5280.
- Zambare,A. S.; Sangshetti ,J. N. ; Kokare,N. D.; Shinde ,D. B. Chinese Chemical Letters 2009, 20,171–174.
- Sarda, S.R.;Jadhav,W. N. ; Pawar,R. P. International Journal of ChemTech Research 2009, 3 ,539-543.
- Bhosalea,R.S. ; Sardaa,S.R.; Girama, R.P.; Rauta,D.S; Parwea, S.P.; Ardhapurea, S.S.Pawarb,R.P. J.Iran.Chem.Soc.,2009,6,519-512 .
- Jae In Lee, Hwa Soo Son, Hyun Park Bull. Korean Chem. Soc. 2004, 25,1945.
- Kumar,S; Sharma ,D. Oriental Journal of Chemistry,2011,27, 761-763.
- Jahangir,G.M. ; Roshani, J.; Scheeren,W. Bulgarian Chemical Communications 2010, 2( 3), 210–216.
- Zhiyun, Du; Huifen N; Kun, Zhang; Zengb,H; Wang, J. Org. Biomol. Chem., 2011, 9, 6930.
- Didier, V.; Messaoud, H. React.Kinet.Catal.Lett.,2001,72,3-10

This work is licensed under a Creative Commons Attribution 4.0 International License.









