Microwave-Assisted One-Step Synthesis of 2,5-Disubstituted-1,3,4-Oxadiazoles Using 1,4- Bis(triphenylphosphonium)-2-Butene Peroxodisulfate
Maryam Gorjizadeh1*, Mozhgan Afshari1 , Simin Nazari2
1Department of Chemistry, Shoushtar Branch; Islamic Azad University, Shoushtar Iran, 64517-41117, Iran 2Department of Chemistry, Sousangerd Branch, Islamic Azad University, Sousangerd, Iran
DOI : http://dx.doi.org/10.13005/ojc/290448
Article Received on :
Article Accepted on :
Article Published : 27 Dec 2013
A series of 1,3,4-oxadiazoles were efficiently synthesized from the cyclization–oxidation reaction of acyl hydrazones by using 1,4-bis(triphenylphosphonium)-2-butene peroxodisulfate as an oxidant in solvent-free medium under microwave irradiation.
KEYWORDS:oxadiazole;thiadiazole;1,4-bis(triphenylphosphonium)-2-butene peroxodisulfate;microwave irradiation;solvent-free synthesis.
Download this article as:| Copy the following to cite this article: Gorjizadeh M, Afshari M, Nazari S. Microwave-Assisted One-Step Synthesis of 2,5-Disubstituted-1,3,4-Oxadiazoles Using 1,4- Bis(triphenylphosphonium)-2-Butene Peroxodisulfate. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Gorjizadeh M, Afshari M, Nazari S. Microwave-Assisted One-Step Synthesis of 2,5-Disubstituted-1,3,4-Oxadiazoles Using 1,4- Bis(triphenylphosphonium)-2-Butene Peroxodisulfate. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1240 |
INTRODUCTION
Since 1960 when Toda et al.,1 first reported the application of solid supports in organic chemistry, the use of this technique has been under investigation.2 Organic oxides such as silica gel, alumina, etc. are used as solid acidic catalysts in a variety of condensation reactions.3 Microwave energy has also developed into a useful technique for a variety of applications in organic synthesis,4 especially for the solvent-free reactions5 since the solvent-free MW-assisted reactions can provide an opportunity to work with open vessels thus avoiding the risk of high pressure development and increasing the potential of such reaction to upscale, and has advantage of rapid reaction rate, high yield and simple work-up procedure.
In recent years 1,3,4-oxadiazole derivatives have received significant attention and have been increasingly investigated due to their diverse range of biological properties. They exhibit, for example, antimicrobial,6 antimycobacterial,7 anticancer,8-9 antiinflammatory,10 carbonic anhydrase inhibiting effect,11 antianxiety, antidepressant.12 Several methods have been reported in the literature for the synthesis of 1,3,4-oxadiazoles. These protocols are routinely multi step in nature. The most general method involves the cyclization of diacylhydrazides with a variety of reagents, such as triphenylphosphine,13 tosylchloride/pyridine14 or phosphorous oxychloride,15-16 usually under harsh reaction conditions. The oxidation of acylhydrazones with different oxidizing agents is another synthetic route to these compounds.17 Few reliable and operationally facile examples have been reported for the one step synthesis of 1,3,4-oxadiazoles, especially from readily available aldehydes and acid hydrazides. However, these protocols use expensive catalysts, and require longer times for the completion of the reactions.
Peroxodisulfate ion is an excellent and versatile oxidant; used mostly for the oxidation of compounds in aqueous solution.18 In spite of the great convenience of using K2S2O8, Na2S2O8 or (NH4)2S2O8 and relatively high oxidation potential, many oxidations by peroxodisulfate do not proceed at a convenient rate. The decomposition of the peroxodisulfate ion requires strong mineral acids and heavy metal ions19 as catalysts and also protic and polar solvents are needed, so the modification of K2S2O8, Na2S2O8 or (NH4)2S2O8 has attracted a great deal of attention.
Recently 1,4- bis(triphenylphosphonium)-2-butene peroxodisulfate (BTPPDS), 20as aninexpensive and environmentally safe oxidation reagent has beenused for synthesis of β-nitrato alcohols, iodination of aromatic compound, and aromatization of Hantzsch 1,4-dihydropyridines in high yields. In this paper, we describe an eco-friendly new method that utilizes BTPPDS on the surface of silica gel as an oxidant for the one-pot synthesis of 2,5-disubstituted 1,3,4-oxadiazoles under solvent- free and microwave irradiation condition.
EXPERIMENTAL
All products were characterized by comparison of their physical data, IR, 1HNMR, and 13C NMR spectra with authentic samples.21-22 1H NMR and 13C NMR spectra were taken on a 400 MHz Brucker Spectrometer. Microwave reactions were conducted in a microwave oven (Milestone-MicroSynth, USA).
1,4-Bis(triphenylphosphonium)-2-butene peroxodisulfate was prepared as described in our previous papers18 and other chemicals were purchased from the Merck Chemical Company, Darmstadt, Germany. The purity determination of the products and reaction monitoring were accomplished by TLC on polygram SILG/UV 254 plates.
General Procedure for the one-pot Synthesis of 1,3,4-Oxadiazoles
The mixture of acyl hydrazide (1 mmol), aromatic aldehyde (1 mmol), BTPPDS (1 mmol) and silica gel (3g) were finely ground with a mortar and pestle. The mixture was then subjected to microwave irradiation in an open Pyrex beaker for 25 min at 450W. Cold water (5 mL) was added and the mixture was extracted with dichloromethane. The combined organic layers solution was dried over MgSO4. The solvent was concentrated in vacuo; the resulting product was recrystallized from dichloromethane to give the desired product (Table 1).
RESULTS AND DISCUSSION
1,4-bis(triphenylphosphonium)-2-butene peroxodisulfatewas readily prepared by adding an aqueous solution of potassium peroxodisulfate to a solution of 1,4-bis(triphenylphosphonium)-2-butene dichloride in water. It is a very stable white solid which can be stored for months without losing its activity. It is soluble in acetonitrile, methanol, dichloromethane, chloroform and ethyl acetate and slightly soluble in CCl4 and diethyl ether.
First, optimization of the reaction conditions for preparation of 1,3,4-oxadiazoles was investigated. Therefore, benzaldehyde (1 mmol) was reacted with acyl hydrazide using different solid supports including silica gel, alumina, and montmorillonite K10. Different combinations of 1,4- bis(triphenylphosphonium)-2-butene peroxodisulfate, and irradiation power were also studied to achieve the maximum chemical yield. From these results, it appeared that when silica gel was the solid support and the microwave power was 450 W, the reaction gave the highest yield within 25 min. According to obtained results, this oxidant acted very efficiently and 1 mmol of the oxidant is enough to convert different aldehydes (1 mmol) carrying electron donating or withdrawing groups to their corresponding products in high isolated yields (Scheme 1, Table 1).
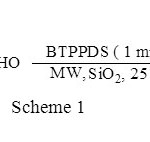 |
Scheme 1: Click here to View figure |
This method appeared to be rapid and economical, with a wide range of applications. The reaction was found to proceed smoothly under microwave irradiation within 25 min whereas under reflux conditions, 12 h were required.19 The products were isolated by simple cold aqueous work-up followed by either solvent extraction or precipitation and were finally purified by column chromatography wherever necessary, to afford pure 2,5- disubstituted 1,3,4-oxadiazole.
Aliphatic aldehydes were also investigated in this reaction but unfortunately, a mixture of compounds was produced and the desired oxadiazoles were obtained in low yields (Table 1, entries 13 and 14).
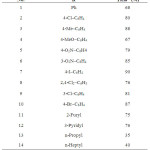 |
Table 1. One-pot synthesis of 2,5-disubstituted 1,3,4-oxadiazolesa Click here to View table |
ACKNOWLEDGEMENTS
We are grateful to the Islamic Azad University Shoushtar Branch for support of this work.
REFRENCES
- Toda, F.; Yagi, M. and Kiyoshigo, K. J. J. Chem. Soc., Chem. Commun., 958(1988).
- Balogh, M. and Laszlo, P.; Organic Chemistry Using Clays, Springer: Berlin, (1992).
- Smith, K. Solid Supports and Catalysts in Organic Synthesis, Ellis Harwood, PTR, Prentice Hall: New York, (1992).
- (a) Mohsenzadeh, F.; Aghapoor, K. and Darabi, H. R.; J. Braz. Chem. Soc. 18, 297(2007), (b) Sena, V. L. M.; Srivastava, R. M.; de Simone, C. A.; Gonçalves, S. M. C.; Silva, R. O. and Pereira, M. A.; J. Braz. Chem. Soc. 18, 1224(2007).
- Heravi, M. M.; Sabaghian, A. J.; Bakhtiari, K.; Ghassemzadeh, M. J. Braz. Chem. Soc. 17, 614(2006).
- Foroumadi, A.; Emani, S.; Hassanzadeh, A.; Rajaee, M.; Sokhanvar, K. and Moshafi M. H. Bioorg. Med. Chem. Lett., 15, 4488(2005).
- Oruc, E .E.; Rollas, S.; Kandemirli, F.; Shvets, N. and Dimoglo, A.; J. Med. Chem. 47, 6760(2004,).
- Matysiak, J.; Nasulewicz, A.; Pelczynska, M.; Switalska, M.; Jaroszewicz, I. and Opolski, A.; Eur. J. Med. Chem., 41, 475(2006).
- H. Baboo and Sahdev., Orient. J. Chem., 29(2), 501-505 (2013).
- Labanauskas, L.; Kalcas, V.; Udrenaite, E.; Gaidelis, P.; Brukstus, A. and Dauksas, A.; Pharmazie, 56, 617(2001,).
- Smaine, F. Z.; Pacchiano, F.; Rami, M.; Barragan-Montero, V.; Vullo, D.; Scozzafava, A.; Winum, J. Y. and Supuran, C.T.; Bioorg. Med. Chem. Lett. 18, 6332(2008,).
- Clerici, F.; Pocar, D.; Guido, M.; Loche, A.; Perlini, V. and Brufani, M.; J. Med. Chem., 44(6), 931(2001).
- Brown, P.; Best, D. J.; Broom, N. J. P.; Cassels, R. P.; Hanlon, J. O.; Mitchell, T. J.; Osborne, N. F. and Wilson, J. M.; J. Med. Chem., 40, 2563(1997).
- Dolman, S. J.; Gosselin, F.; O’Shea, P. D. and Davis, I. W.; J. Org. Chem., 71, 9548(2006).
- Khan, K. M.; Ullah, Z.; Rani, M.; Perveen, S.; Haider, S. M.; Choudhary, M. I.; Rahman, A. U. and Voelter, W.; Lett. Org. Chem., 1, 50(2004,).
- A. Bidram, F. Hatamjafari and A. Doryeh., Orient. J. Chem., 29(1), 123-126 (2013)
- Dabiri, M.; Salehi, P.; Baghbanzadeh, M. and Bahramnejad, M.; Tetrahedron Lett. 47, 6983(2006). (b) Rostamizadeh, S.; and Housaini, S.; Tetrahedron Lett. 45, 8753(2004,).
- House, D. A. Chem. Rev., 62, 185(1962).
- Anderson, J. M. J. Am. Chem. Soc., 92, 1651(1970).
- (a) Badri, R.; Gorjizadeh, M. Synth. Commun. 39, 4239(2009), (b) Badri, R.; Gorjizadeh, M. Chin. Chem. Lett. 20, 1439(2009), (c) Gorjizadeh, M. and Abdollahi-Alibeik, M.; Chin. Chem. Lett. 22, 61(2011).
- Badri, R. and Gorjizadeh, M.; Phosphorus, Sulfur, and Silicon, 185, 544(2010).
- Al-Omar, M.; Al-Deeb, O.A.; and Al-Khamees, H.A. Phosphorus, Sulfur, and Silicon, 179, 2509(2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.









