Design and synthesis of a new androgen derivative using some strategies
1Figueroa-Valverde Lauro*, 2Díaz-Cedillo Francisco, 1García-Cervera Elodia, 1Pool-Gómez Eduardo, 1López-Ramos Maria, 3Rosas-Nexticapa Marcela, 1Hau-Heredia Lenin, 4Sarabia Alcocer B, 1Zepeda-Acosta B.
1Laboratory of Pharmaco-Chemistry, Faculty of Chemical Biological Sciences, University Autonomous of Campeche, Av. Agustín Melgar s/n, Col Buenavista C.P.24039 Campeche Cam., México. 2Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional. Prol. Carpio y Plan de Ayala s/n Col. Santo Tomas, México, D.F. C.P. 11340. 3Facultad de Nutrición, Universidad Veracruzana, Médicos y Odontologos s/n C.P. 91010, Unidad del Bosque Xalapa Veracruz, México. 4Faculty of Medicine, University Autonomous of Campeche, Av. Patricio Trueba de Regil s/n, Col Lindavista C.P.24090 Campeche Cam., México.
DOI : http://dx.doi.org/10.13005/ojc/290403
Article Received on :
Article Accepted on :
Article Published : 11 Jan 2014
Anew androgen derivative was synthesized using some strategies; in the first stage the compound of N-(1,10-phenanthrolin-5-ylmethyl)ethane-1,2-diamine (3)was obtained by the reaction of 1,10-phenanthroline with ethylenediamine in presence of formaldehyde. The second stage was achieved by the reaction of 3 with testosterone using boric acid as catalyst to form the compound 10,13-dimethyl-3-{2-[([1,10]phenanthrolin-5-ylmethyl)-amino]-ethylimino}-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-ol (5). Finally, the compound 7 (Chloro-acetic acid 3-((3-chloro-2-oxo-cyclobutyl)-{2-[(3-chloro-2-oxo-cyclobutyl)-[1,10]phenanthrolin-5-ylmethyl-amino]ethyl}-amino)-10,13-dimethyl-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl ester was synthesized by thereaction of 5 with chloroacetyl chloride in presence of triethylamine. The structure of the compounds obtained was confirmed by elemental analysis, spectroscopy and spectrometry data. The proposed method offers some advantages such as good yields, simple procedure, low cost, and ease of workup.
KEYWORDS:1,10-phenanthrolin;testosterone;boric acid
Download this article as:| Copy the following to cite this article: Lauro F. V, Francisco D. C, Elodia G. C, Eduardo P. G, Maria L. R, Marcela R. N, Lenin H. H, Sarabia A.B, Zepeda-Acosta B. Design and synthesis of a new androgen derivative using some strategies. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Lauro F. V, Francisco D. C, Elodia G. C, Eduardo P. G, Maria L. R, Marcela R. N, Lenin H. H, Sarabia A.B, Zepeda-Acosta B. Design and synthesis of a new androgen derivative using some strategies. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1583 |
Introduction
There are several reports on development of some androgen derivatives; for example, the synthesis of 17α-(tributylstannylethynyl)-4-androsten-17-ol-3-one by the reaction of 17-ethynyl-4-androsten-17-ol-3-one with Bu3SnOMe to reflux1. Other data indicate the preparation of 3-oxoandrost-4-en-17β-yl-2-methyl-1H-imidazole-1-carboxylate by the reaction of 17-hydroxyandrost-4-en-3-one-1,1-carbonylbis(2-methylimidazole) in anhydrous acetonitrile2. In addition, other study showed the synthesis of (17S)-spiro-3,3-(dimethoxy)-5α-androstan-17β,2´-oxirane by the reaction of 3,3-(dimethoxy)-5α-androstan-17-one with trimethylsulfonium iodide in DMF3. There is other reportwhich shown the synthesis of the dehydroisoandrosterone derivative by the reaction between a brucine derivative anddehydroisoandrosterone 3-sulfate using boric acid as catalyst4. Other data indicate the preparation of 17-(2-[[(2-hydroxy-naphtalen-1-yl)phenyl-methyl]-amino]ethylamino)-10,13-dimethyl-2,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol by the reaction between 1-[(2-amino-ethylamino)- phenyl-methyl]-naphthalen-2-ol and androsterone using boric acid as catalyst5. Additionally, other androgen (Succinic acid mono-{6-[(2-amino-ethylamino)-methyl]-1-ethinyl-10a,12a-dimethyl-2,3,3a,3b,4,5,10,10a,10b,11,12,12a-dodecahydro-1H-7-oxa-8-aza-dicyclopenta- [a,h]-phenanthren-1-yl}ester) was prepared by reaction of hemisuccinate of danazol with ethylenediamine hydrochloride in presence of formaldehyde6.Also Other report7 indicate the preparation of 17β-(N-Acetyl-4′-imidazolyl)-3β-acetoxyandrost-5-ene by the reaction of 17β-(4′-Imidazolyl)androst-5-en-3β-ol in pyridine/Ac2O.All these experimental results show several procedures which are available for synthesis of diverse androgen derivatives; nevertheless, expensive reagents and special conditions are required. Therefore, in this study anandrogen derivative was synthesized using some strategies.
EXPERIMENTAL
The compounds evaluated in this study were purchased from Sigma-Aldrich Co. Ltd. The melting points for the different compounds were determined on an Electrothermal (900 model). Infrared spectra (IR) were recorded using KBr pellets on a Perkin Elmer Lambda 40 spectrometer. 1H and 13C NMR spectra were recorded on a Varian VXR-300/5 FT NMR spectrometer at 300 and 75.4 MHz in CDCl3 using TMS as internal standard. EIMS spectra were obtained with a Finnigan Trace GCPolaris Q. spectrometer. Elemental analysis data were acquired from a Perkin Elmer Ser. II CHNS/0 2400 elemental analyzer.
N-(1,10-phenanthrolin-5-ylmethyl)ethane-1,2-diamine (3)
A solution of 1,10-phenanthroline (100 mg, 0.55 mmol), ethylenediamine (100 µl, 1.50 mmol) and 10 ml of formaldehyde was stirred for 72 h to room temperature. The reaction mixture was evaporated to a smaller volume. After the mixture was diluted with water and extracted with chloroform. The organic phase was evaporated to dryness under reduced pressure, the residue was purified by crystallization from methanol:water (3:1) yielding 85 % of product, m.p. 98oC; IR (Vmax, cm-1): 3382, 3310,1540;1H NMR (300 MHz, CDCl3) dH: 2.40 (broad, 3H), 2.69(t, 2H, J =6.0), 2.83 t, 2H, J =6.0), 3.53 (m, 2H), 7.27-8.12 (m, 4H),8.18-8.78 (m, 2H), 8.87 (d, 1H, J = 4.0 Hz) ppm. 13C NMR (75.4 MHz, CDCl3) dC: 41.50, 53.12, 53.48, 120.90, 123.17, 127.50, 129.15, 130.42, 133.91, 135.02, 141.08, 144.10, 145.12, 148.20, 152.03 ppm. EI-MS m/z: 252.12 (M+10). Anal.Calcd.for C15H16 N4: C, 71.40; H, 6.39; N, 22.21. Found: C, 71.38; H, 6.36.
10,13-dimethyl-3-{2-[([1,10]phenanthrolin-5-ylmethyl)-amino]-ethylimino}-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-ol(5)A solution of 3 (100 mg, 0.40 mmol), testosterone (118 mg, 0.40 mmol) and boric acid (90 mg, 1.45 mmol) in 10 ml of methanol was stirred for 72 h to room temperature. The reaction mixture was evaporated to a smaller volume. After the mixture was diluted with water and extracted with chloroform. The organic phase was evaporated to dryness under reduced pressure, the residue was purified by crystallization from methanol:water (4:1) yielding 62 % of product, m.p. 80-82oC; IR (Vmax, cm-1): 3410, 3324, 3312; 1H NMR (300 MHz, CDCl3) dH:0.80 (s, 3H), 0.92-1.00 (m, 2H), 1.02 (s, 3H), 1.06-1.40 m, 6H), 1.56-1.62 (m, 4H), 1.86 (m, 2H), 2.04-2.38 (m, 5H), 3.04 (t, 2H, J = 6.44 Hz), 3.50 (t, 2H, J = 6.44 Hz), 3.68 (m, 1H), 4.10 (t, 2H, J = 6.44 Hz), 4.70 (broad, 2H), 5. 97 (d, 1H, J = 1.84), 7.20-7.80 (m, 7H) ppm. 13C NMR (75.4 MHz, CDCl3)dC: 11.13 3, 17.75, 20.75, 23.38, 30.11, 30.27, 30.4, 32.98, 33.21, 35.58, 36.64, 37.86, 50.61, 50.97, 51.01, 51.73, 53.16, 80.72, 115.5, 120.95, 124.35, 127.54, 129.51, 133.18, 134.09, 136.84, 141.08, 145.18, 146.49, 148.29, 155.11, 157.83, 165.96 ppm.EI-MS m/z: 522.30 (M+12). Anal. Calcd. for C34H42N4O: C, 78.12; H, 8.10; N, 10.72; O, 3.06. Found: C, 78.10; H, 8.08.
Chloro-aceticacid 3-((3-chloro-2-oxo-cyclobutyl)-{2-[(3-chloro-2-oxo-cyclobutyl)-[1,10]phenanthrolin-5-ylmethyl-amino]ethyl}-amino)-10,13-dimethyl-2,3,6,7,8,9,10,11, 12, 13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl ester (7).
A solution of 5 (200 mg, 0.38 mmol), triethylamine(100 µl, 1.50 mmol) and chloroacetyl chloride (128 µl, 1.60 mmol) in 10 ml of methanol was stirred for 72 h to room temperature. The reaction mixture was evaporated to a smaller volume. After the mixture was diluted with water and extracted with chloroform. The organic phase was evaporated to dryness under reduced pressure, the residue was purified by crystallization from methanol:water (4:1) yielding 70 % of product, m.p. 122 oC; IR (Vmax, cm-1): 1738, 1705, 1208;1H NMR (300 MHz, CDCl3) dH: 0.76 (m, 1H), 0.80 (s, 3H), 0.86 (m, 1H), 1.00 (s, 3H), 1.06-1.18 (m, 2H), 1.50-1.66 (m, 7H), 1.69 (m, 1H), 1.72 (m, 1H), 1.80 (m, 1H), 1.88-1.90 (m, 2H), 1.96 (m, 1H), 2.06 (m,1H), 2.08 (m, 1H), 2.10-2.60 (m, 4H), 2.62-2.90 (m, 4H), 3.60 (m, 1H), 3.72-3.76 (m, 2H), 3.98 (t, 1H, J = 15.94 Hz), 4.08 (t, 2H, J = 15.94 Hz), 4.30 (t, 1H, J = 15.94 Hz), 4.60 (m, 2H), 4.80 (m, 1H), 5.01 (d, 1H), 7.20-8.80 (m, 7) ppm.13C NMR (75.4 MHz, CDCl3) dC: 12.00, 18.86, 20.64, 23.80, 27.53, 28.25, 29.66, 32.30, 32.49, 32.76, 33.35, 35.80, 35.92, 37.44, 40.80, 42.63, 50.02, 51.76, 53.07, 56.00, 57.28, 59.02, 62.88, 62.96, 69.77, 71.44, 81.44, 120.38, 121.45, 124.08, 127.54, 128.88, 130.90, 133.18, 134.12, 140.45, 144.41, 145.02, 146.20., 148.12, 155.33, 168.14,201.34, 202.22 ppm.EI-MS m/z: 804.26 (M+12). Anal. Calcd. for C44H51Cl3N4O4: C, 65.55; H, 6.38; Cl, 13.19; N, 6.95; O, 7.94. Found: C, 65.52; H, 6.34.
Results and discussion
There are many procedures for preparation of several androgen derivatives; nevertheless, despite its wide scope, have some drawbacks; for example, several agents used have limited stability and their preparation require special conditions8-10. Analyzing these data, we reporta straightforward route for synthesis of an androgen derivative using some strategies. The first stage was achieved by the synthesis of N-(1,10-phenanthrolin-5-ylmethyl)ethane-1,2-diamine (3). It is important to mention that there are reports which show the condensation of some compounds with formaldehyde and amino groups; for example, the synthesis of an androgen derivative (6-[(1-Ethynyl-1-hydroxy-10a,12a-dimethyl-2,3,3a,3b,4,5,10,10a,11,12,12a,dodecahydro-1H-7-oxa-8-aza-dicyclopenta[a,h]phenanthren-6-yl-methyl)-amino]hexanoic acid) by the reaction of danazol and 6-aminohexanoic acid in presence of formaldheyde6. Therefore, in this study 3 was prepared by the reaction of 1,10-phenanthroline with ethylenediamine in presence of formaldehyde (Figure 1). The1H NMR spectrum of 3 shows signals at 2.40 ppm for amino groups; at 2.69 and 2.83 ppm for arm bound phenanthroline fragment; at 3.53 ppm for methylene group bound to both amino and phenyl groups; at 7.27-8.87 ppm for phenanthroline fragment. The 13C NMR spectrum of 3 contains at 41.50 and 53.48 ppm for methylene groups involved in the arm bound to phenanthroline fragment; at 53.12 ppm for amino group bound to phenyl group; at 120.90-152.03 ppm for phenanthroline fragment. Finally, the presence of compound 3was further confirmed from mass spectrum which showed a molecular ion at m/z 252.12.
f1 f2 f3
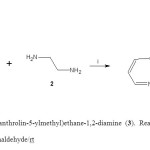 |
Figure 1. Synthesis of N-(1,10-phenanthrolin-5-ylmethyl)ethane-1,2-diamine (3). Reaction of 1,10-phenanthroline (1)with ethylenediamine (2) to form 3. i = formaldehyde/rt Click here to View figure |
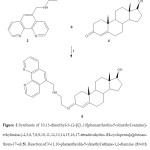 |
Figure 2. Synthesis of 10,13-dimethyl-3-2-[([1,10]phenanthrolin-5-ylmethyl)-amino]-ethylimino-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenan- thren-17-ol (5). Reaction of N-(1,10-phenanthrolin-5-ylmethyl)ethane-1,2-diamine (3)with testosterone (4) to form 5. ii = boric acid/rt. Click here to View figure |
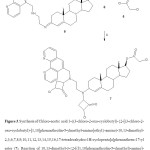 |
Figure 3.Synthesis of Chloro-acetic acid 3-((3-chloro-2-oxo-cyclobutyl)-2-[(3-chloro-2-oxo-cyclobutyl)-[1,10]phenanthrolin-5-ylmethyl-amino]ethyl-amino)-10,13-dimethyl-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl ester (7). Reaction of 10,13-dimethyl-3-2-[([1,10]phenanthrolin-5-ylmethyl)-amino]-ethylimino-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenan- thren-17-ol (5) with chloroacetyl chloride(6) to form 7. iii = triethylamine/rt. Click here to View figure |
The second stage was achieved by reaction of the compound 3 with testosterone (Figure 2) resulting in imino bond formation involved in the compound 5(10,13-Dimethyl-3-{2-[([1,10]phenanthrolin-5-ylmethyl)-amino]-ethylimino}-2,3,6,7,8,9,10,11,12,13,14,15,16, 17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-ol).It is noteworthy thatthere are several proceduresfor the synthesisofiminogroups are previously described intheliterature11-13;for example the synthesis of a steroid derivative by the reaction of N1-(2,3-dimethoxystrychnidin-10-yliden)-ethane-1,2-diamine with a steroid derivative using boric acid as catalyst4. For this reason, in this study this reagent was used as catalyst in the reaction of the compound 3 with testosterone (Figure 1) to form the compound 5.The1H NMR spectrum of 5 shows signals at 0.80 and 1.02 ppm for methyl groups; at 0.92-1.00, 1.06-2.38, 3.68 and 5.90 ppm for steroid nucleus; at 3.04 and 3.50 ppm for methylene groups bound to both imino and amino groups; at 4.10 ppm for methylene group bound to both phenyl and amino groups; at 4.70 ppm for both amino and hydroxyl groups; at 7.00-7.80 for phenanthroline fragment. Finally, the presence of compound 5was further confirmed from mass spectrum which showed a molecular ion at m/z 522.30.
The third stepwas achieved bythe synthesisof7(Chloro-acetic acid 3-((3-chloro-2-oxo-cyclobutyl)-{2-[(3-chloro-2-oxo-cyclobutyl)-[1,10]phenanthrolin-5-ylmethyl-amino]ethyl}-amino)-10,13-dimethyl-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl ester)byreactionof 5with chloroacetylchloride in the presenceoftriethylamine.This method has been previously reported for other type of compound withbothaminoand iminogroupsinvolved in its structure chemical, which react with chloroacetylchloride to form cyclobutanone groups14.However, it isnoteworthy that hydroxylgroupof compound 5 was esterified with acetylchloride. This phenomenon is similar to esterification of other type of compounds15,16. The1H NMR spectrum of 7 shows signals at12.00 and 18.86 ppm for methyl groups; at 20.64-29.66. 32.76-37.44, 42.63, 51.76, 56.00, 59.02, 81.44, 121.45 and 146.20 ppm for steroid nucleus; at 32.30, 32.49, 62.88-71.44 ppm for cyclobutanone groups; at 40.80 ppm for methylene group bound to both ester and chloride groups; at 50.02 and 53.07 ppm for methylene groups bound to both amino groups; at 57.28 ppm for methylene group bound to both amino and phenyl groups; at 120.38, 124.45-145.02 and 148.12-155.33 ppm for phenanthroline group; at 168.14 ppm for ester group; at 201.34 and 202.22 ppm for ketone groups. Finally, the presence of compound 7was further confirmed from mass spectrum which showed a molecular ion at m/z 804.26.
CONCLUSIONS
In this study, we reported an efficient andsimple method for synthesis of anandrogen derivative. It is important to mentionthat this method is highly versatile and the yield isgood.
References
- Top S., Thibaudeau C., Vessieres A., Brulé E., Le-Bideau F. and Joerger J. Organometallics. 28:1414-1424 (2009).
- Moreira V., Vasaitis T., Guo Z., Njar V. and Salvador J. Steroids.73:1217-1227 (2008).
- Mappus E., Chambon C., Fenet B., Ravel M., Grenot C. andCuilleron C. Steroids. 65:459-481 (2000).
- Figueroa-Valverde L., DÍaz-Cedillo F., García-Cervera E., Pool Gómez E., López-Ramos M., Rosas-Nexticapa M. andMartinez-Camacho R. Arch. Pharm. Res. 36:1270-1278 (2013).
- Figueroa-Valverde L., DÍaz-Cedillo F., García-Cervera E., Pool Gómez E., Rosas-Nexticapa M. andLópez-Ramos M.Asian J.Chem.25: 6724-6726 (2013).
- Figueroa-Valverde L., DÍaz-Cedillo F., García-Cervera E., Pool Gómez E. andLópez-Ramos. E-J Chem. 7:S191-S196 (2010).
- Yang-zhi L., Ji-song L., Yang L., Katsuya K., Klus G. and Brodie A. J. Med. Chem.40: 3297-3304 (1997).
- Herr M. and Heyl F. J. Am. Chem. Soc.75:5927-5930 (1953).
- Stevenson D., Neville J.and Akhtar M. J. Chem. Soc. Chem. Commun.1078-1080 (1985).
- Potter G., Banie E., Jarman M. and Rowlands M. J. Med. Chem. 38:2463-2471 (1995).
- Shirayev A., Moiseev I. and Karpeev S.Arkivok.4:199-207 (2005).
- Uppiah U., Bhowon M. and Jhaumeer S.E-J Chem.6:S195-S200 (2009).
- Hania M.E-J Chem.6:629-632 (2009).
- Figueroa-Valverde L., DÍaz-Cedillo F., Rosas-Nexticapa M., García-Cervera E., Pool Gómez E. and López-Ramos M.Oriental J. Chem. 29:921-925 (2013).
- Martin A., Sanchez-Chaves M., Arranz F. Reac.Func.Polym.39:179-187(1999).
- Bai F., Li R., Yang X. and Li S.Polymer Int. 55:319-325 (2006).

This work is licensed under a Creative Commons Attribution 4.0 International License.









