A facile and Convenient Method for Synthesis of Thiiranes under Mild Condition Using Phase Transfer Catalyst
Maryam Gorjizadeh1*, Mozhgan Afshari1
1Department of Chemistry, Shoushtar Branch, Islamic Azad University, Shoushtar Iran, 64517-41117, Iran
DOI : http://dx.doi.org/10.13005/ojc/290454
Article Received on :
Article Accepted on :
Article Published : 16 Jan 2014
A mild and efficient process for preparation of thiiranes with KSCN in water at room temperature using a catalytic amount of 1,4-bis(triphenylphosphonium)-2-butene dichloride is described.
KEYWORDS:Epoxide; thiirane; 1,4-bis(triphenylphosphonium)-2-butene dichloride; phase transfer catalysts.
Download this article as:| Copy the following to cite this article: Gorjizadeh M, Afshari M. A facile and Convenient Method for Synthesis of Thiiranes under Mild Condition Using Phase Transfer Catalyst. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Gorjizadeh M, Afshari M. A facile and Convenient Method for Synthesis of Thiiranes under Mild Condition Using Phase Transfer Catalyst. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1721 |
INTRODUCTION
Epoxides are among the most useful synthetic intermediates towards the synthesis of many biologically active compounds1 and a large variety of reagents are known for the ring opening of these compounds.2 Their electrophilic reactions with different nucleophilic anions have been an interesting subject in organic synthesis.3 Of these anions, the reaction of thiocyanate ion with epoxides has been widely studied and is a suitable method for the preparation of
thiiranes.4 Other reagents such as phosphine sulfide,5 methylbenzothiazol-2-thione,6 and dimethylthio formamide7 have been reported to produce thiiranes from oxiranes. Usually with these sulfurating agents, several different Lewis acids such as RuCl3,8 BiCl39 and TiO(CF3COO)210 or a protic acid like TFA5 oroxalic acid 11 have been employed as catalysts. More recently the β-cyclodextrin complexes12 and cyanuric chloride13 have been used for the conversion of oxiranes to thiiranes. However many of these methods suffer from using strongly acidic or oxidizing conditions, undesirable side reactions, long reaction time, high temperature, use of organic solvents and desulfuration of the resulting thiirane to the olefin.
The toxic and volatile nature of many organic solvents, particularly chlorinated hydrocarbons, that are widely used in organic synthesis have posed a serious threat to the environment. Consequently methods that successfully minimize their use are the focus of much attention. Water as a solvent is safe, economical and environmentally benign.14 In view of the advantages of using water as a solvent, we explored the synthesis of various types of thiiranes from oxiranes and potassium thiocyanate in presence of 1,4-bis(triphenylphosphonium)-2-butene dichloride as an efficient PTC in water at room temperature.
Phase transfer catalysts (PTCs) are powerful reagents in chemical transformations,15 the characteristics of which include mild
reaction conditions, safety, operational simplicity and selectivity. PTCs are often used in nucleophilic displacement reactions to facilitate reactions between organic reactants and ionic inorganic salts. Although many phase transfer catalysts are known, Phosphonium salts are practically important and used in many of organic reactions.
EXPERIMENTAL
All products were characterized by 1H NMR, 13C NMR, IR and by comparison with authentic samples. 6,9,16 The IR spectra were recorded on Bomem FT-IR spectrometer. 1H NMR and 13C NMR spectra were taken on a 400 MHz Broucker spectrometer. The C, H, P analyses were performed by the microanalytical service of research institute of petroleum industry (N. I. O. C). Melting points were measured on a mettler FP5 apparatus. 1,4-bis(triphenylphosphonium)-2-butene dichloride was prepared and other chemicals were purchased from the Merck chemical company Darmstadt, Germany. The purity determination of the products and reaction monitoring were accomplished by TLC on polygram SILG/UV 254 plates.
Preparation of 1,4-Bis(triphenylphosphonium)-2-butene Dichloride (BTPBDC)
To a solution of 1,4-dichlorobutene (0.625g, 5 mmol) in CHCl3 (10 mL) in a 50 mL round-bottomed flask equipped with a magnetic stirrer and a reflux condenser was added triphenylphosphine (2.62g, 10 mmol). The reaction mixture was refluxed on a water bath for 2.5 h. The solution was cooled to room temperature and then, while vigorously stirred, diethylether was added dropwise until an oily product separated. The ether was removed by decantation and acetone (40 mL) was added. Stirring the acetone solution for 40 min afforded a white precipitate which was filtered, washed with acetone (20 mL) and dried. Yield 2.596g (80%), m.p. 278-279 °C. IR (KBr):υ = 3053(m), 2755(w), 1613(m), 1575(s), 1478(s), 1437(s), 1258(s), 754(s), 693(s), 556(s) Cm-1. 1H NMR(CDCl3): δ = 5.7 (dd, JPH=15.7, JHH=8, 4H), 6.3 (m, 2H), 7.7-8.0 (m, 30H). 13C NMR, (CDCl3): δ = 20.7, 120.8, 132.15, 134.2, 137.01, 140.17. Anal. Calcd: C, 73.055; H, 5.5396; P, 9.5421. found: C, 73.9599; H, 5.5469; P, 9.5531%.
Conversion of oxiranes to thiiranes: General Procedure
The epoxide (1 mmol), potassium thiocyanate (1 mmol), BTPBDC (0.2 mmol) and water (10 mL) were stirred at room temperature for an appropriate time. The progress of each reaction
was monitored by TLC. The reaction mixture was extracted with diethylether (3×10 mL). The organic solution was concentrated in vacuo, the resulting product was directly charged on a small silica gel 60 (0.2-0.5 mm) column 25 Cm and eluted with a mixture of ethylacetate and n-hexane (1:4) to afford the pure thiirane.
RESULTS AND DISCUSSION
1,4-bis(triphenylphosphonium)-2-butene dichloride [BTPBDC] was easily prepared by reacting 1,4-dichlorobutene with triphenylphosphine in chloroform under reflux condition (Scheme 1). The reaction is very clean and phase transfer catalyst can be easily precipitated in acetone.
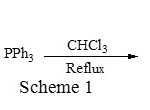 |
Scheme 1 Click here to View figure |
We examined the catalytic ability of BTPBDC for conversion of epoxides to thiiranes. Therefore, phenyl glycidyl ether (1 mmol) was reacted with potassium thiocyanate (1 mmol) in the presence of BTPBDC in different solvents. The results in Table 1 were clearly shown water was the best solvent among those tested. TLC analysis was showed at room temperature, conversion was completed after 2.5 h and corresponding thiirane was produced in 87% isolated yield.
According to obtained results, this catalyst acted very effciently and it was observed that only 0.2 M equivalent of the catalyst is enough to convert different epoxides carrying electron donating or with-drawing groups to their corresponding thiiranes in high isolated yields (Scheme 2, Table 2).
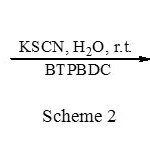 |
Scheme 2 Click here to View figure |
It is noteworthy that no evidence for the formation of olefins as by-product of the reaction was observed. In organic solvents; the reaction did not complete after 3 h and was contaminated by diol and thiocyanohydrine formation. In the absence of catalyst, the conversion of oxiranes was very slow and the yields were also very low (20% in 6 h). It is believed that BTPBDC can catalyze the formation of thiiranes from epoxides in the aqueous phase in two ways. Firstly by swelling in the aqueous media it can act as phase transfer catalyst for the the epoxides, and can therefore provide the necessary microenvironment for the water-soluble nucleophile, that is, SCN–, to be in the vicinity of the epoxides for the reactions to occur. Secondly, the phosphonium functionalities on these catalyst can interact as a Lewis acid with the oxygens of the epoxides to facilitate the attack of the thiocyanate anion as the nucleophile. It is
worthy to note that the reaction medium was almost neutral, sensitive functionalities such as carbon-carbon double bonds remain intact.
 |
Table 1. Reaction of different epoxides with KSCN using BTPBDC as catalyst Click here to View table |
In conclusion, we have developed a novel, eco-friendly, and efficient protocol for the synthesis of thiiranes with KSCN using 1,4-bis(triphenylphosphonium)-2-butene dichloride as a catalyst. This
method offers several advantages including mild reaction conditions, high conversions, short reaction times and high isolated yields. It can be emphasized that the reaction is clean and from economical and environmental points of view, use of water as solvent is favorable than organic solvents.
ACKNOWLEDGEMENTS
We are grateful to the Islamic Azad University Shoushtar Branch for support of this work.
REFRENCES
- Corey, E.J.; Shibata, S.; Bakshi, R.K. J. Org. Chem. 53, 2861(1988).
- (a) Iranpoor, N.; Baltork, I.M. Synth. Commun. 20, 2789(1990). (b) Cha, J.S.; Park, S.J. Bull. Korean Chem. Soc., 30, 2823 (2009). (c) Kim, Y.S.; Lee, Y.; Kim, G.J. Bull. Korean Chem. Soc. 30, 1771(2009).
- Iranpoor, N.; Kazemi, F.; Salehi, P. Synth. Commun. 27, 1247(1997).
- Tamami, B.; Kiasat, A.R. Synth. Commun., 26, 3953(1996).
- Chan, T. H.; Finkenbine, J. R. J. Am. Chem. Soc. 2880(1972).
- a) Calo, V.; Lopez, L.; Marchese, L.; Pesce, G. J. Chem. Soc., Chem. Commun. 62(1975). (b) Cambie, R. C.; Mayer, G. D.; Rutledge, P. S.; Woodgate, P. D. J. Chem. Soc., Perkin Trans. 1 52(1975).
- Takido, T.; Kobayashi, Y.; Itabashi, K. Synthesis 779(1986).
- Iranpoor, N.; Kazemi, F. Tetrahedron 53, 11377(1997).
- Mohammadpoor-Baltork, I.; Alian, H. Synth. Commun. 28, 3943(1998).
- Iranpoor, N.; Zeyni-Zaadeh, B. Synth. Commun. 28, 3913(1998).
- Kazemi, F.; Kiasat, A. R. Phosphorus, Sulfur, and Silicon, 178, 1333(2003).
- Surendra, K.; Krishnaveni, N.S.; Rao, R.S. Tetrahedron Lett. 45, 6523(2004).
- Bandgar, B.P.; Joshi, N.S.; Kamble, V.T.; Tetrahedron Lett. 47, 4775(2006).
- Trost, B. M. Angew. Chem., Int. Ed. Engl. 34, 259(1995).
- (a) Takido, T.; Toriyama, M.; Yamashita, K.; Suwa, T.; Seno. M. Phosphorus, Sulfur, and Silicon 178, 319(2003). (b) Weng, Z. H.; Wang, J. Y.; Jian, X. G. Chin. Chem. Lett. 18, 936(2007).
- Iranpoor, N.; Kazemi, F. Synthesis, 821(1996).

This work is licensed under a Creative Commons Attribution 4.0 International License.









