Uptake of Nutrients in Vegetables Grown on FGD-Gypsum-Amended Soils
Yutdanai Yodthongdee1, Ponlayuth Sooksamiti2, Jaroon Jakmunee1 and Somchai Lapanantnoppakhun1*
1Department of Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand 2Office of Primary Industries and Mines, Region 3 Chiang Mai 50300, Thailand
DOI : http://dx.doi.org/10.13005/ojc/290324
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
This research evaluated the effects of using flue gas desulphurization gypsum (FGDG) for growing of some agronomic crops. The FGDG was added to soil at 0, 2.5, 5.0 and 7.5% by weight. The test plants, Chinese kale and green bean, were grown and harvested after 45 days and 60 days, respectively. Application of FGDG at all ratios significantly increased pH of the soil, due to the lime containing in FGDG. The heavy metals content in plants grown in the FGDG treated tanks were not significantly different from those of the control tank. From the ten studied elements in Chinese kale and green bean seed tissues (As, Ca, Cd, Cr, Cu, K, Mg, Na, Pb, and Zn), the content of five toxic elements (As, Cd, Cr, Cu, and Pb) were very low and not significantly influenced by FGDG, while the content of some nutrient elements (K, Ca, Mg) in the plant tissues growing in FGDG treated soil were higher than the control. Concentration of some micronutrients (Cu and Zn) in plants decreased with increasing dose of FGDG. There has not been any negative effect from applying up to 5.0% FGDG in soil. The results showed possibility of using FGDG as soil amendment in terms of agricultural production and safety.
KEYWORDS:FGDG;Vegetable;Nutrients;Heavy metals
Download this article as:| Copy the following to cite this article: Yodthongdee Y, Sooksamiti P, Jakmunee J,Lapanantnoppakhun S. Uptake of Nutrients in Vegetables Grown on FGD-Gypsum-Amended Soils. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290324 |
| Copy the following to cite this URL: Yodthongdee Y, Sooksamiti P, Jakmunee J,Lapanantnoppakhun S. Uptake of Nutrients in Vegetables Grown on FGD-Gypsum-Amended Soils. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=306 |
Introduction
Lignite is the important fuel for electricity production in Thailand. The reserved amounts are high and it is economical and socially acceptable. Flue gas desulfurized gypsum (FGDG) is a major by-product of various types of lignite power plants resulting from scrubbing process for reduction of flue gas discharge sulfur dioxide into the atmosphere. FGDG mainly contains SO2 reaction products such as gypsum (CaSO4・2H2O).
In Thailand, the biggest thermal power generated plant is in Northern region, it uses lignite as a combustible fossil fuel. The average sulfur content of lignite mines is 3.2%. Approximately 17.5 million tons of lignite, are supplied to the power generating unit. Over 500,000 tons of sulfur dioxide were emitted, when the plant operated at full covering without emission contact [1,2].
At present, due to the increase of power demand the amount of FGDG from the power plant increases continuously. This leads to the shortage of on-site dump storage space and environmental problem to the surrounding area and it has a negative impact on aquatic and terrestrial systems through runoff [3,4]. Nevertheless, FGDG is relatively high purity [5], it is higher in calcium and sulfur and contains lower amounts of heavy metals. Considering, the main chemical composition of FGDG, it has been used as an economical conditioner for agriculture soils [3, 4, 6]. But one side effect of FGDG application is the enhancement of leaching of some metal ions and environmentally sensitive
elements in soil. Because the level of trace elements content in lignite fuels is determined by the difference in the quality of the coal origin, its rank and geological history. Although FGDG has been shown to increase crop product, it may contain certain trace elements at injurious level; to plants and the food-chain [7,8]. Some researchers have used FGDG as soil amendments in non-alkali soil. Nevertheless, FGDG is an excellent source of micronutrients essential for plant growth, particularly boron (B) and sulfur (S) [9-11].
Several studies have grown a variety of plants on FGDG amended soils in pot or field studies in order to investigate the effect of FGDG in soils on heavy metal availability and metal uptake by plants (corn, wheat, grass, cane, tomato etc.) [14-15]. But information about the effects of FGDG on phytoavailability of metals of vegetables is limited [16]. Farina and Channon (1988), Shainberg et al. (1989), and Pavan and Bingham (1986) showed that the applications of gypsum significantly increased calcium, and sulfur levels, while magnesium content was significantly decreased [17-19]. Information about FGDG effects on plant growth needs to be evaluated if it will be used for soil amendment. The objective of the present work is to study the effect of FGDG application to soil on the uptake and bioaccumulation of some nutrient metals and heavy metals in some vegetables (Chinese kale and green beans). Soil was mixed with various amounts of FGDG as a source of plant nutrients and subjected to mesocosms experiment (tank experiment) [20]. The aim of the research is to evaluate the feasibility of using FGDS as soil amended material for agricultural production.
MATERIALS AND METHODS
FGDG and soil sample
FGDG samples were collected from the lignite power plant, Lampang province. Sample collection was carried out from dump waste, obtaining third different samples that were mixed and homogenized to give a single sample. Those samples were analyzed for their elemental compositions by Atomic Absorption Spectrometric techniques [5]. The paddy field soils at 0-to 20-cm depth were collected from TakFa district, Nakornsawan province (Soil 1) and Samko district, Ang-thong province (Soil 2) and used to make the various amendments for experimental studies. The soils in these areas are generally characterized by low water availability and this can alter the rate of organic matter mineralization and thus the availability of trace metals. The samples were transported in polyethylene bags to laboratory and air-dried. After drying, the soil samples were ground in a hammer mill and passed through 2 mm sieve.
Tank experiments
The experiment consisted of the evaluation of 3 different FGDG treatments (0(control); 2.5, 5.0 and 7.5 %) by weight added to soil separately and mixed thoroughly with the soil samples. The mixed soils incubation were carried out for 30 days in a greenhouse in a completely randomized distribution for each soil at ambient temperature and free ventilation [20]. The soils were irrigated to keep the moisture by spraying water. After that, the mixed soils were conducted in round cement tanks (80 I.D. X 50 cm height). Each tank was filled with the soil samples to 30 cm depth. In each tank Chinese kales or green bean seedlings with two leaves were initially planted and only healthier plants were remained in each tank (Figure 1). All the plants were watered daily and moisture with tap water for the duration of the experiment. Soil samples were taken from tank for chemical analysis. The elemental compositions of soils are summarized in Table1.
 |
Figure 1. A tank experiment Click here to View figure |
After 45 days of growing for the Chinese kale and 60 days for green bean, they were harvested. Plant samples were harvested from the tank by cutting the plants 2 cm above the soil surface and kept in separate polyethylene bags. Afterwards, the non-edible parts were removed and the samples were thoroughly washed first with tap water and then followed by distilled water. Separated the above-ground portion and then oven-dried at 60oC to constant weight. The dried materials were ground using a hand mortar and stored in paper bags for further analysis.
Sample preparation
Plant digestion[21]
The dry ground samples were accurately weighed 1.0 – 2.0 g and put into the tall beaker. Added 20 mL of concentrated nitric acid and 1.0 mL of 30% w/v hydrogen per oxide and pre-digested in a fume hood over night. Place the beaker on the hot plate and digest at 80 oC 2 – 3 hours or until obtaining clear solution. Each sample was digested and analyzed in duplicate. After digestion and cooling, added 20 mL of deionized water and then filtered by using whatman paper No 40 and diluted to 100 mL with deionized water in a volumetric flask.
Soil digestion[21,22]
Soil analyses were performed on samples from all tanks involved in the study. An air-dried soil sample was ground in a mortar and well mixed. Accurately weight (1.0 g) of each sample was taken into a pyrex beaker, three replicates for each sample, and added 12 mL of aqua regia (3 ml HNO3 + 9 ml HCl) solution after that kept overnight in a fume hood for predigestion. The solutions were heated on the hot plate until fuming and taken near dryness. After cooling, the solution was adjusted to 10 mL by using 1% v/v HNO3 and filtered through filter paper (Whatman No. 5). The filtrate was adjusted the volume to 50 mL in a volumetric flask by using 1% v/v HNO3.
For quality control spiked soil and plant samples were used through all analysis. Both plants and soil-digested sample solutions were analyzed by flame atomic absorption spectrophotometer (FAAS) NOV AA 350, Analytik Jena, Germany, Electro thermal atomic absorption spectrophotometer (ETAAS), AAnalyst 800, Perkin Elmer, USA and hydride generation atomic absorption spectrophotometer (HGAAS) HS 60 Hydride system NOV AA 350, Analytik Jena, Germany.
Results and Discussion
pH and Metals analysis of Soil
FGDG application significantly increased the overall soil pH from 6.60, 5.44 to 6.91 and 6.65 respectively (Table II). This increase would typically be expected given the portion of unreacted lime in the FGDG. Because this FGDG was obtained from the desulfurization unit installed in lignite power plants, they contained small quantities of heavy metals originating in the fly ash produced by lignite combustion. Therefore, one concern of using FGDG as soil amendment is the potential toxicity of the heavy metals to plants and their accumulation in the food chain. The content of metals (As, Ca Cr, Cu, Cd, Fe, Hg, Mn, Mg, Na, Ni, K, Pb, Se and Zn) in FGDG is presented in Table I. Heavy metals contents in FGDG are lower than those that found in the soils (Table II)
![TABLE I: Major, minor and trace elements in FGDG [5]](http://www.orientjchem.org/wp-content/uploads/2013/11/Vol29_No3_yy_uptake_t1-150x150.jpg) |
TABLE I: Major, minor and trace elements in FGDG [5] Click here to View table |
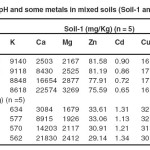 |
TABLE: II pH and some metals in mixed soils (Soil-1 and Soil-2) Click here to View table |
The Table II showed that the amount of sodium, potassium and some heavy metals in treated soils were not significantly different from the control soil. It indicated that there was no contribution of these metals from FGDG. However, the amounts of Ca and Mg in all treated soils were increased with the increase of FGDG amounts. It indicated that they came from FGDG.
Metals Content in Plants
By using FGDG, the soil’s physical and chemical properties may change and they affected to the absorption of each metal by plants. There are many factors affecting micronutrient availability, such as soil pH, organic matter and etc. The FGDG was added to soil at 2.5, 5.0 and 7.5% by weight.
It was found that all rates of FGDG application significantly increased pH of the soil. The metal content in plants grown in each tank was measured and compared with that in plants grown in the control tank. The ten elements, i.e, As, Ca, Cd, Cr, Cu, K, Mg, Na, Pb, and Zn in Chinese kale tissues and Green bean seed tissues were monitored. The results are shown in Table III. It was found that five toxic elements (As, Cd, Cr, Cu, and Pb) in plants were not significantly influenced by FGDG. As can be seen from the results, heavy metals (As, Cd, Cr, Cu and Pb) contents in plants grown in the gypsum-treated tanks were not significantly different from those in the control tank. This indicates that the gypsum treatment did not result in an increase of metals in the plants. The treatment of FGDG to soil at 5.0% or higher resulted in the higher concentrations of Ca, Mg and K in plants than those of the control case (approximately 9.2%, 3.7% and 5.0% higher, respectively for 5.0% FGDG treatment versus non-treatment). It indicated that these three nutrient elements came from FGDG and the FGDG could be used as a source of nutrient for plants.
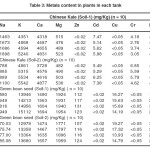 |
TABLE III: Metals content in plants in each tank. Click here to View table |
Conclusion
FGDG can be used as treatment material for soil amended. FGDG can release Ca and Mg to soil. Ca, Mg and K which releasing from FGDG can be uptake by Chinese kale and Green bean. Contents of heavy or toxic metals in the FGDG are low and they did not contaminate in soil and studied plants. Therefore, there have not been any negative effects from applying up to 5.0% FGDG in soil. Addition of FGDG to the soil is not only increase total concentration of the some macronutrients for soil but also the bioavailable pool of these elements for plant.
Acknowledgments
We gratefully acknowledge Office of Primary Industries and Mines, Region 3 Chiang Mai for providing research facility and The Graduate School Chiang Mai University for partial support.
References
- J. Kamyath. (March 2003). Thai technology. Eng. Today. [Online]. 1(3). Available:http://www.engineeringtoday.net/magazine/articledetail.asp?arid=539&pid =74
- V. Punyawadee, R. Pothisuwan, N. Winichaikule and K. Satienperakul, “Economy and Environment Program for Southeast Asia,” EEPSEA Research report, no. 2006-RR4, October 2006.
- Y. B. Lee, J. M. Bigham and P. J. Kim, “Evaluate Changes in Soil Chemical Properties Following FGD-Gypsum Application,” Korean J. Env. Agri., vol. 26, no. 4, pp. 294-299, November 2007.
- R. B. Clark, S. K. Zero, K. D. Ritchey and V. C. Baligar, “Growth of forages on acid soil amended with flue gas desulphurization by-products,” Fuel, vol. 76, No. 8, pp. 771–775, January 1997.
- Y. Yodthongdee, P. Sooksamiti and S. Lapanantnoppakhun, “Mineralogical and Chemical Properties of Flue Gas Desulphurization Gypsum from Lignite-Fired Power Plant, Thailand,” Paccon 2012, Chiang Mai Univ., Thailand, January 2012.
- S. Chun, M. Nishiyama and S. Matsumoto, “Sodic soils reclaimed with by-product from flue gas desulphurization: corn production and soil quality;” Environ. Pollut., vol. 114, no. 3, pp. 453-459, 2001.
- Donstova et al. “Agricultural Uses for Flue Gas Desulfurization (FGD) Gypsum,” USEPA., EPA530-F-08-009, March 2008.
- A. K. Pendias, “Trace Elements in Soils and Plants”, 3rd ed., CRC Press, Boca Raton, Washington, D.C, 2001.
- A. K. Alva, O. Prakash and S. Paramasivam, “Flue-gas desulfurization gypsum effects of leaching of magnesium and potassium from Candler fine sand,” Commun. Soil Sci. Plant Anal, vol. 29, pp. 459–466, 1998.
- J. T. Crews and W. A. Dick, “Liming acid forest soils with flue gas desulfurization by-product: growth of Northern red oak and leachate water quality,” Environ. Pollut, vol 103, pp. 55–61, July 1998.
- J. J. Sloan, R. H. Dowdy, M. S. Dolan and G. W. Rehm, “Plant and soil responses to field-applied flue gas desulfurization residue”, Fuel, vol. 78, pp. 169–174, February 1999.
- Y. B. Lee, J. M. Bigham and P. J. Kim, “Evaluate Changes in Soil Chemical Properties Following FGD-Gypsum Application”, Korean J. Env. Agri., vol. 26, no. 4, pp. 294-299, December 2007.
- S. Narra, “Flue Gas Desulphurization Gypsum as a Soil Amendment in the Growth of Wild Rye and Poplar(Hybrid 275 and Weser 6) Clones in Lusatia, Germany”, Int. J. Agric. Res., 2009.
- C. Kampichler, A. Bruckner and E. Kandeler, “Use of enclosed model ecosystems in soil ecology: a bias towards laboratory research”. Soil Biol. Biochem., vol. 33, pp. 269-275, 2001.
- Y. Saka, S. Matsumoto and M. Sadakata, “Alkali soil reclamation with flue gas desulfurization gypsum in China and assessment of metal content in corn grains”, Soil Sed. Contam., vol. 13, pp. 65–80, 2004.
- R. B. Clark and V. C. Baligara, “Growth of Forage Legumes and Grasses in Acidic Soil Amended with Flue Gas Desulfurization Products”, Commun., Soil Sci. Plant Anal., vol. 34, no. 1 & 2, pp. 157–180, 2003.
- M. P. W. Farina, P. Channon and G. R. Thibaud, “A comparison of strategies for ameliorating subsoil acidity I. Long term growth effects”, Soil Sci. Soc. Am. J., vol. 64, no. 2, pp. 646-651, March 2000.
- M. A. Pavan, F. T. Bingham and P. F. Pratt, “Redistribution of Exchangeable Calcium, Magnesium, and Aluminum Following Lime or Gypsum Applications to a Brazilian Oxisol”, Soil Sci. Soc. Am. J., vol. 48, pp. 33-38, January 1984.
- A. S. Knox, J. D. Knox, D. C. Adriano and K. S. Sajwan, “Influence of Coal Combustion Flue Gas Desulfulfurization Waste on Element Uptake by Maize (Zea Mays L.)”, Paper No. DE-AC09-96SR18500, U. S. Department of Energy, 2005.
- C. Lin, W. Lu, Y. Liu, Y. Ma, F. Wan and A. Chen, “Effects of chemical amendment on heavy metal leachability and bioavailability of an acidic mine water-contaminated soil and growth of a vegetable”, 2010 19th World Congress of Soil Science, Soil Solutions for a Changing World, August 2010.
- Standard Operating Procedure for the Digestion of Solid Samples Using Method 3050B Hotplate/Beaker Digestion Technique, SW-846 Method 3050B (Hotplate, Beaker, Soil/Sediment), Metals 034, United States Environmental Protection Agency, USA, 1999.
- VGB Powertech, GmbH, “Instruction Sheet Analysis of FGD gypsum” VGB – M701, 2nded. 2008.

This work is licensed under a Creative Commons Attribution 4.0 International License.









