Synthetic and Structural Studies of some Co (II), Ni (II) and Cu (II) Complexes with Nitrogen and Sulphur containing Schiff Base
Chitranjan Prasad Choudhry, S. P. Sharma, C.L. Rai and B. K. Rai
1Department of Chemistry, Veer Kuwer Singh University Ara, India. 2Department of Chemistry S.V.P. College, Bhabhua, India. 3Department of Chemistry, S.B. College Ara, India. *4Department of Chemistry, L.N.T. College, Muzaffarpur, R.A. Bihar University, India.
DOI : http://dx.doi.org/10.13005/ojc/290315
A Schiff base derived from 1-propyl 2-6-diphenyl piperidone thiosemicarbazone (PDPT) and its Co(II), Ni(II) and Cu(II) transition metal complexes were synthesized. Structure of the ligand and its complexes were derived on the basis of various physicochemical techniques such as molar mass, elemental analyses, Infrared spectra, electronic spectra, magnetic susceptibility and molar conductivity. On the basis of these observations, it has been observed that the ligand PDPT, coordinate to the metal ion in a bidentate fashion through the nitrogen and sulphur atom of thiosemicarbazone moiety. The remaining co-ordination centres are satisfied by anions such as Cl-, Br-, and I-. Electronic spectra and magnetic susceptibility measurements reveal octahedral geometry for the Co(II) and Ni(II) ions where distorted octahedral geometry for Cu(II) complexes. The complexes were found to be non-electrolytic in nature on the basis of low value of molar conductance.
KEYWORDS:PDPT/ Co(II);Ni(II) and Cu(II)/ Schiff base;Thisemicarbazone;complex
Download this article as:| Copy the following to cite this article: Choudhry C. P, Sharma S. P, Rai C. L, Rai B. K. Synthetic and Structural Studies of some Co (II), Ni (II) and Cu (II) Complexes with Nitrogen and Sulphur containing Schiff Base. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290315 |
| Copy the following to cite this URL: Choudhry C. P, Sharma S. P, Rai C. L, Rai B. K. Synthetic and Structural Studies of some Co (II), Ni (II) and Cu (II) Complexes with Nitrogen and Sulphur containing Schiff Base. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=286 |
INTRODUCTION
Schiff base ligand containing nitrogen and sulphur show broad biological activity1-10 due its different ways in which they are bonded to metal ions. The Schiff base ligands are vital tool for the chemist as there are several biocidal and industrial applications. Metal complexes of nitrogen and sulphur containing ligand have attracted considerable attraction because nitrogen and sulphur play key role in many metallic bio-molecules. Schiff bases show different biological applications such as, anti-fungal, anti-bacterial and anti-tumour activities. In recent year Schiff base ligands and their metal complexes have attracted greater interest in medicinal bio-organic and pharmaceutical field. Keeping the above facts in mind ant in continuation of our recent11 work in this field we report here the synthesis and characterization of Co(II), Ni(II) and Cu(II) complexes with ligand 1-propyl 2- 6- diphenyl piperidone thiosemicarbazone.
EXPERIMENTAL
All the chemicals used were of analytical grade. Metal contents were determined using standard method16. The analytical data, colour, molar conductivity, magnetic susceptibility, decomposition temperature and electronic spectral measurements have been recorded in Table-1. IR spectra of the ligands and complexes were recorded on Perkin Elmer model 577 spectrophotometer using KBr disc. Magnetic susceptibility was carried out by Gouy method using Hg[Co(NCS)4] as calibrant. Electronic spectra were recorded on cary-2390. Spectrophotometer using DMF as a solvent. Molar conductance of the complexes was measured using Systronic conductivity meter model 303 in DMF.
Synthesis of the Schiff Base
The ligand PDPT was prepared by the condensation of 1-propyl-2-6-diphenyl pepridone dissolved in minimum amount of ethanol with semi-carbazide hydrochloride in 1:1 molar ratio. The mixture was refluxed on a water bath for 3 and half hour. The resulting colourless solution was concentrated to half of its original volume and allowed to crystallize with ethanol. The crystal formed were collected after filtration and dried in oven. Yield 65%; m.p.- 183+1oC.
Preparation of the complexes
Hot ethanolic solution (20ml) of the corresponding metal halides(0.01mol) were mixed with a hot ethanolic solution (20ml) of the ligand(0.02mol). The reaction mixture was refluxed on water bath for 4 hour on cooling, a coloured compound was precipitated out in each case. The compound was filtered, washed with cold ethanol and dried in oven yield-65%.
RESULTS AND DISCUSSION
The interaction of Co(II), Ni(II) and Cu(II) metal halides with ligand PDPT result in the formation of complexes have the general composition [M(PDPT)2X2]where M= Co(II), Ni(II) and Cu(II), X= Cl–, Br–, and I–.
Infrared spectra
The characteristic IR band (4000-200cm-1) for the free ligands when compared to those of its Co(II), Ni(II) and Cu(II) complexes provide meaningful information regarding the bonding sites of the ligand.(Table-2). It is established from the survey of the literature that thiosemicarbazone ligand may coordinate as bidentate ligand, through azomethine nitrogen and thione sulphur. The IR spectra of the ligand exhibit strong and broad band at 1680cm-1 assignable17 to nC=N. This broad band undergoes red shift after complex formation; Proposes linkage through azomethine nitrogen. The next IR spectra of ligand exhibit strong and broad band at 800cm-1 assignable17 to nC=S. This band is also shifted to lower frequency region in the complexes proposes coordination of the thione sulphur atom to the metal ions. The coordination through azomethine N and thione sulphur of thiosemicarbazone moiety is further supported by the appearance of band in the far IR regions at 420-395 cm-1 and 480-455 cm-1 assigned17 to nM–N and nM–S respectively.
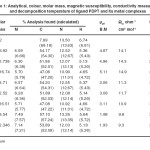 |
Table-1: Analytical, colour, molar mass, magnetic susceptibility, conductivity measurement and decomposition temperature of ligand PDPT and its metal complexes Click here to View table |
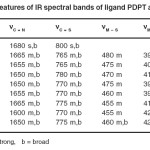 |
Table – 2: Salient features of IR spectral bands of ligand PDPT and its complexes Click here to View table |
![Fig.1 [M(PDPT)X2]](http://www.orientjchem.org/wp-content/uploads/2013/11/Vol29_No3_chitran_syn_fig1-150x150.jpg) |
Fig.1 [M(PDPT)X2] Click here to View figure |
The linkage with halogen are indicated by the appearance of the another band in the far IR region at 320-260cm-1 assignable17 to nM–X. (X= Cl–, Br– or I–). The coordination with halogen with metal ion supported by the low molar conductance of the complexes of the complexes in the range 9.3-14.9 ohm-1 cm2 mol-1. The electronic spectral18 and magnetic Susceptibility19-20 measurements proposes octahedral geometry for the complexes which is justified by other physiochemical as well as infrared spectral data.
Molar Conductance
The observed molr conductance of Co(II), Ni(II) and C(II) complexes in 10-3 M DMF solution in the range 9.3-14.9 W-1 cm2 mol-1. The molar conductance values are consistent with non-electrolytic nature21 for all metal complexes.
Conclusion
Infrared data of the ligand and metal complexes suggest, N, S and halogen coordination of the ligand. The analytical data correspond to [M(PDPT)ZX2] and molar conductance in DMF are consistent with non-electrolytic behavior on the basis of above physiochemical investigation the proposed structure is given in Fig-1.
REFERENCES
- Farghaly A.A., Bekhit A.A. and Park Young Ji, Pharm. Med. Chem., 53, 333(2000).
- M. Nadeemsiddiqui, Arshad, M. Faiz, Ahsan Waquar and Alam M. Shamser, Int.J. Pharm. Sci.,Dry, Res; 1, 136, 143(2009).
- Vigato P.A. and Tamburini S., Coord. Chem. Rev; 248, 717(2004).
- Roy P., Dhara K., Manassero O. M. and Banerjee, Inorg. Chem. Commun; 11, 265(2008).
- Kulkarni N.V., Satisha M.P., Budagumpi S., Kurdekar and Revankar V.K., J. Coord. Chem; 63, 1451(2010).
- Chakrborty J., Nandi M., Mayer Figgie H., Sheldric W.S., Sorane L., Bhaymik A. and Banerjee P., Eur. J. Inorg. Chem; 32, 5033(2007).
- Abu-Hussen, A.A.A., J. Coord. Chem., 59, 157(2006).
- Karthikeyan M.S., Prasad D.J., Poojary B., Bhatt K.S., Holla B.S. and Kumar N.S., Bioorg. Med. Chem.; 14, 7482(2006).
- Singh K. Barwa M.S. and Tyagi P., Eur. J. Med. Chem, 41, 1(2006).
- Paneerselvam P., Nair R.R., Vijaylakshmi G., Subramaniam E.H., Sridhar S.K., Eur. J. Med. Chem.; 40, 225(2005).
- Rai B.K. and Anand Rahul, Asian J. Chem., 25, 583(2013).
- Rai B.K., Thakur Amrita and Divya, Asian J. Chem.; 25, 583(2013).
- Rai B.K. and Vidyarthi S.N., kumara Punam, Kumari Suman, Kumari Lakshmi and Singh Rajkishore, Asian J. Chem., 25, 941(2013).
- Rai B.K. and Kumar Arun, Asian J. Chem., 25, 1160(2013).
- Rai B.K., J. Indian Chem., Soc; 90, 105(2013).
- Vogel A.I., A textbook of quantitative Chemical Analysis, Revised by Menaham J., Denny R.C., Barnes J.D. and Thomas M, Pearson Education, 7th Edn, London(2008).
- Silverstein R.M. and Webster F.X., Spectrometric Identification of Organic Compounds, 6th edn, John Wiley and Sons, 109(2008). Kemp William, Organic Spectroscopy, Polgrave, New York, 3rd Edn.(2008).
- Lever A.B.P., Inorganic Spectroscopy, Elsecier, New York(1960).
- Carlin R.L. and Van Deynevetedt A.J., Magnetic properties of transition metal compounds springer-ver lag, New York, (1997).
- Figgis B.N., Introduction to LIgand Field, Wiely Eastern Ltd., New Delhi, 279(1976).
- Geary W.J., Coord. Chem., Rev; 7, 81(1971).

This work is licensed under a Creative Commons Attribution 4.0 International License.









