Synthesis and Characterization of 2-imino-3–(2-Hydroxylphenyl)-1-Thiazolidin-4-one Substituted Ammine Complexes of Cr(III), Co(III), Ni(II) and Cu(II)
Abadi Hadush, R.K. Upadhyay* And Tesfahun Kebede
Department of Chemistry, College of Natural and Computational Sciences Haramaya University, Ethiopia.
DOI : http://dx.doi.org/10.13005/ojc/290361
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
Mixed ligand complexes of Cr(III), Co(III), Ni(II) and Cu(II) synthesized by partial substitution of 2-imino-3–(2-hydroxylphenyl)-1-thiazolidin-4-one in respective ammine complexes were characterized by elemental analysis, conductance and magnetic measurements, infrared and uv-visible spectroscopy. Cr(III), Co(III) and Ni(II) complexes were octahedral whereas Cu(II) complex was square planar.
KEYWORDS:Mixed-ligand complexes;ligand - substitution reaction;thiazolidinone
Download this article as:| Copy the following to cite this article: Hadush A, Upadhyay R. K, Kebede T. Synthesis and Characterization of 2-imino-3–(2-Hydroxylphenyl)-1-Thiazolidin-4-one Substituted Ammine Complexes of Cr(III), Co(III), Ni(II) and Cu(II). Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290361 |
| Copy the following to cite this URL: Hadush A, Upadhyay R. K, Kebede T. Synthesis and Characterization of 2-imino-3–(2-Hydroxylphenyl)-1-Thiazolidin-4-one Substituted Ammine Complexes of Cr(III), Co(III), Ni(II) and Cu(II). Orient J Chem 2013;29(3). Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=384 |
INTRODUCTION
Many metal complexes of biologically important ligands are more effective than their free ligands1. Mixed ligand complexes have a pivotal role in biological chemistry2 as these create specific structures3 and have been implicated in the storage and transport of active substances through membranes. Among these ligands are thiazolidnones, derivatives of thiazolidine. Plenty of thiazolidnone derivatives have been found to exhibit profound broad spectrum of biological properties, viz. bactericidal4, fungicidal5, anti-convalsant6, anti-inflammatory7, antitumor8, anti-HIV9 etc. Although reports on synthesis, characterization and biological properties of mixed ligand complexes derived either by direct interaction10-14 of metal salts with two/three organic donor molecules or by substitution15 of strong ligand(s) in weakly coordinated ligand of complexes are plenty, ligand exchange reactions16,17 involving replacement of strongly coordinated ligands like ammonia, cyanide and thiocyanide by organic ligands such as Schiff’s bases alone or alongwith other ligands like thiourea and/or anions are scantly but surprisingly no reference is available on the chemistry of substitution products of thiazolidnone ligands in strongly coordinated ammine complexes hitherto.
In view of high biological significance of mixed ligand complexes of biologically important ligands and non-availability of any report on the thiazolidnone substituted ammine complexes as yet, it has been thought worthwhile to synthesize and characterize mixed ligand complexes by substitution of 2-imino-3–(2-hydroxylphenyl)-1-thiazolidin-4-one in ammine complexes of Cr(III), Co(III), Ni(II) and Cu(II) and report in the present communication.
EXPERIMENTAL
Materials
Chloroaceylchloride, ortho-aminophenol and potassium thiocyanate BDH/ALDICH reagents of 97-99%, purely were used in the synthesis of 2-imino-3–(2-hydroxylphenyl)-1-thiazolidin-4-one (ligand). All the metal chlorides were Analytical grade (BDH) products and used as supplied without further purification.
Physical measurements and analysis
Melting points of the ligand and complexes were determined in open glass capillaries and are uncorrected. The infrared spectra were recorded on Prestige-21 FT-IR spectrophotometer in 4000cm-1– 400cm-1 range in KBr medium. Electronic spectra of complexes were recorded in ethanol on ITEM No -275 spectrophotometer in 200-800cm-1 range. Magnetic susceptibility measurements were done on MSB-AUTO, (Sherwood Scientific) magnetic balance. The molar conductivity measurements were carried out using Jenway digital conductivity meter. Carbon, hydrogen, nitrogen and sulphur analyses were done on microanalytical Perkin- Elmer 240C elemental analyzer whereas metal analysis was carried out on an atomic absorbtion spectrometer, Buck Scientific Model 210 VGP AAS. The presence of chloride is established by decomposing the complexes and treating with AgNO3.
Synthesis of ligand
The ligand, 2-imino-3–(2-hydroxylphenyl)-1-thiazolidin-4-one, was synthesiszed by reported method in two steps. In the first step ortho hydroxyphenyl chloroacetanilide , the precursor of the ligand, was synthesized by adding 35 ml chloroacetatylchloride dropwise to 0.37 mol of orthoamino phenol in benzene, the precipitate of the product was filtered, washed with benzene and dried in air. In the second step, a mixture containing 2-hydroxyphenyl chloroacetanilide (0.18 mol) and potassium thiocyanate (0.195 mol) in acetone was refluxed for 5 h. The reaction mixture was filtered after cooling at room temperature and the solvent of filtrate was evaporated on water bath. Finally residue was washed with water repeatedly and dried in air and crystallized from alcohol.
Synthesis of mixed ligand complexes
Ammine complexes of Cr(III)19, Co(III)20, Ni(II)21 and Cu(II)22 were synthesized by reported methods. With the saturated solution of each of the ammine complex (0.15 mol) in alcohol-water (2:3, v/v) saturated solution of ligand (0.30 mol) in alcohol was mixed and 5 ml concentrated ammonia was added. This reaction mixture was refluxed for 1 h. During refluxing ammonia solution was occasionally added to the reaction mixture to compensate any loss of ammonia. The precipitates obtained, on cooling of the reaction mixtures, were filtered out and washed with ice cold alcohol and dried in air. Purity of the synthesized compounds was checked by TLC.
RESULTS AND DISCUSSION
Analytical and conductivity data of the complexes are presented in table 1. The analytical data correspond to a metal- ligand – ammonia ratio of 1:1:1. The conductivity values in ethanol correspond to 1:1 electrolyte for Cr(III) complex while Co(III), Ni(II) and Cu(II) complexes are non-electrolytes.
The infrared spectra of mixed ligand complexes synthesized are compiled in table 2. The infrared spectra of these complexes in comparison with free ammonia and free ligand show characteristic band positions and shifts which can be correlated to monodentate ammine binding and bidentate chelation respectively. Besides, metal binding through water molecules is also evident from the IR spectra. Free ammonia displays17 ѵN-H band in the region 3300-3500 cm-1. In the present complexes IR spectra show characteristics bands in the region 3173-3250 cm-1 which are lower compared to those of free ѵNH3. Hence it can be concluded that the nitrogen of ammonia is involved in coordination. The bands occurring at 508 cm-1, 495 cm-1, 522 cm-1 and 523 cm-1 in Cr(III), Co(III), Ni(II) and Cu(II) complexes respectively, attributed to ѵM-NH3, strongly support the presence of ammonia in coordination zone of metals.
As regards chelation through 2-imino-3–(2-hydroxylphenyl)-1-thiazolidin-4-one ligand, IR spectra exhibit significant features in ѵO-H (phenolic) and ѵC-N-C (heterocyclic’s) regions. The disappearance of ѵO-H frequency of free ligand (3410 cm-1) and negative shift in ѵC-N-C (1329 cm-1) in complexes are clear evidence of coordination of deprotnated phenolic oxygen and heterocyclic ring nitrogen with various metal ions. Weak energy bands occurring in 550-610 cm–1 and 462-508 cm-1 regions of the IR spectra corresponding to ѵM-O and ѵM-N respectively substantiate the involvement of deprotnated oxygen of phenolic group and pentacyclic nitrogen of the ligand. Slightly positive changes in ѵN-H (azomethine) in complex spectra ruled out coordination of azomethine nitrogen.
Lattice water displays symmetrical and asymmetrical stretching and bending vibrations in 3317-3375 cm-1 and 1618-1664 cm-1 regions respectively. The broad peak structure shows overlapping of azomethine ѵN-H bands. The water of coordination exhibits its characteristic ρr, ρt, ρw vibrations in 837-916 cm-1 range of IR spectra.
The magnetic data pertaining to these systems are depicted in table 3. The magnetic moment of Cr(III) complex is consistent with the spin- free d3 octahedral geometry involving d2sp3 hybridization. The magnetic moment of 2.86 BM of Co(III) complex is very unusual. This value neither corresponds to spin-paired octahedral system nor to a spin – free configuration of the metal in the complex. The observed magnetic moment indicates spin-crossover from high – spin
(µs 4.89 BM) to low – spin (µs 0.00 BM) or vice versa. The Ni(II) complex displaying magnetic moment 2.65 BM, close to high spin d8 octahedral spin-only value of 2.83 BM with two unpaired electrons. The magnetic moment 1.85 BM of Cu(II) complex is indicative of one unpaired electron in this system.
The electronic spectral data of the complexes are noted in table 3. In the electronic spectra of Cr(III) complex three d-d transition bands observed at 20940 cm-1, 24400 cm-1 and 28570 cm-1 corresponding 26 to 4A2g → 4T2g (F), 4A2g → 4T1g (F), 4A2g → 4T1g (P) spin-allowed transitions respectively are consistent with octahedral stereochemistry of this complex. The electronic spectra of Co(III) complex displays multiple bonds. First band at 15450 cm-1 is assigned 27 to 1A1g → 3T2g(G) spin-forbidden transition whereas other two bands at 20964 cm-1 and 22716 cm–1 correspond to 1A1g →1T1g(G) and 1A1g → 1T2g(G) spin-allowed transitions respectively of octahedral geometry. Fourth band of high energy attributed to charge transfer from ligand to metal. The electronic spectra of Ni(II) complex show multiple bands, First three of these bands are assigned 28,29 to spin allowed transitions 3A2g → 3T2g , 3A2g → 3T1g (F) and 3A2g → 3T1g (P) respectively of spin-free d8 system. Fourth band of high energy corresponds to charge transfer. First three band of lower energy in the electronic spectra of Cu(II) complex are characteristic 30 of 2B1g → 2A1g (dx2– y2 → dz2), 2B1g → 2B2g (dx2– y2 → dzy), and 2B1g → 2Eg d-d transitions of square planar geometry, two high energy bands correspond to charge transfer.
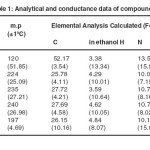 |
Table 1.Analytical and conductance data of compounds Click here to View table |
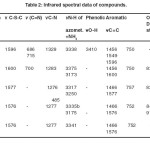 |
Table 2. Infrared spectral data of compounds. |
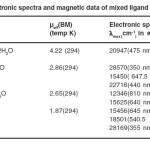 |
Table 3.Electronic spectra and magnetic data of mixed ligand complexes Click here to View table |
ACKNOWLEDGEMENT
Financial support from the Haramaya University, Ethiopia is gratefully acknowledged.
REFERENCES
- Jona, E., Kubranova, M., Simon, P., Mrozinski, J., J. Thermal Analysis. 46: 1325(1996)
- Mildvan, A.S., Cohn, M., J. Biological Chemistry. 241:1178-1193(1960)
- Bouwman, E., Driessen,W.L., Redijk, J., Coordination Chemistry Reviews. 104:143-172(1990)
- B.K. Rai, S.N. Vidyarthi, P. Singh, K.C. Singh, S. B. Sahi and J.S. Ojha, Orient J. Chem., 28(3): 1365-1370 (2012).
- Sayyed, M., Mokle, S., Bokhare, M., Mankar, A., Surwase, S., Bhusare, S., Vibhutea,Y., Akrivoc, ii: 187-192(2006)
- Valts,V., Upadhyay, R.K., Sharma, P., J. Theoretical and Applied Sciences. 1:24-26(2009)
- Velmurugan, V., Leelavathi, N., Kalvikkarasi, S., Priya, S.S., Anandhi, V.M., J. Chem. Teach. Research. 4:01-04(2012)
- Goel, B., Ram, T., Tyagi, R., Bansal, E., Kumar, A., Mukherjee, D., Narayan, J. S., J.Med.Chem. 34:265-269(1999)
- Wang, S., Zhao,Y., Zhang, G., LV,Y.,Gong, P., J.Medical Chemistry.46:3509-3518(20110)
- Letizia, M. B., Chimirri, A., De, L. L., Monforte, A. M., Monforte, P., Rao, A., Zappala, M., Balzarini, J., De, E.C., Pannecouquec, C, Witruow, M., Bioorganic and Medical Chemistry, 11:1793-1796(2001)
- Wang, G. P., Cheng, L., Zhu, L.G., Chines Chemical letters, 14:1182-1184(2003)
- Found, D.M., Bayoumi, A., El-Gahami, M.A., Ibrahim, S.A., Hammam, A. M., J. Natural Scienc. 2:817-827(2010)
- El-Said, A. I., Aly, A. A., El-Meligy, M.S., Ibrahim, M.A., J. Aregentine chemical society. 97:149-165(2009)
- Reddy, P.R., Reddy, M., Proc.Indian Acad.Sci.(Chem.Sci.) 112:593-600(2000)
- Reddy, R., Sudhakar, K., Indian J. Chem. 30:1182(1991)
- M.H. Salunke, Z.A. Filmwala and A.D. Kamble, Orient J. Chem., 27(3): 1243-1248 (2011).
- Upadhyay, R. K. J. Indian Chem.Soc. 74: 214(1997)
- Upadhyay, R. K. J. Indian Chem.Soc.75:535(1997)
- Upadhyay, R.K., Bajapi, A. K., Rathore, K., Transtion Met.Chem. 10:24(1985)
- Lakhay, R., Singh, R. L., J. Agric.Food.Chem.39:580-583(1999)
- Bramwell, F.B., Dillard, C.R., Shahani, C.J., Wieder, G.M., Investigations in General Chemistry. Burgess, Minneapolis,146-153(1977)
- Nordmann, J., Kuljian, E.S., Quantitative Freshman Chemistry. Burgess, Minneapolis ,106-109(1966)
- Kirubel,T., Chemistry of p-dimethylaminoanil of o-hydroxyphenylglyoxal substituted ammine complexes of Cr(III), Co(III), Ni (II), Cu(II) and Zn(II). M.Sc. thesis (2012)
- Schlessinger, G.G., Inorganic Laboratory Preparations Chemical Publishing, New York, 194(1962)
- Bellamy, L. J., The infrared spectra of complex molecules (London: Chapman and Hall) 3rd edn. (1975)
- Nakamoto, K., Infrared spectra of inorganic and coordination compounds (New York Wiley Interscience) (1970)
- Upadhyay, R. K., Rani, A., Acta Chimica Hungarica, 126: 195(1989)
- Thaker, B.T., Lekhadia, J., Patel, A., Thaker, P., Trans. Met. Chem. 19: 623( 1994)
- Douglas, B., McDaniel, D., Alexander, J., Concepts and Models of Inorganic Chemistry, Wiley, NewYork . A respected inorganic Chemistry text. 3rd ed. (2004)
- Lewis, J., Wilkins, R. G., Modern coordination Chemistry (New York: Interscience) (1967)
- Reddy, P.R., Reddy, A.M., Proc. India Acad.Sci (Chem.Sci), 112: 593-600(2000)
- Upadhyay, R.K., D.Sc. Thesis, C.C.S. Univ.(1994)

This work is licensed under a Creative Commons Attribution 4.0 International License.









