Studies on Leaching of Heavy Metals from E – waste
Caroline Michael1 and R.Wilfred Sugumar2
1Department of Physics, Madras Christian College (Autonomous) 2Department of Chemistry, Madras Christian College (Autonomous), Tambaram, Chennai 600 059, India.
DOI : http://dx.doi.org/10.13005/ojc/290343
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
One of the global problems in the present day world is the negative impacts created by the growing e-waste on the natural resources like air, soil and water. In the present work, the discarded electronic components were allowed to leach in water samples drawn from different rivers and the leachate were analyzed for the presence of heavy metals. The results highlight that even if the contact time is short, the toxic elements tend to leach to a great extent. In our studies, Arsenic had leached to an extent of 0.053ppm, Cadmium 0.010 ppm, Chromium 0.029 ppm, Lead 0.042ppm and Mercury 0.061ppm. The results indicate that most of the metals have a tendency to leach and the extent of leaching depends on the quality of the water. As a result of leaching the physico-chemical parameters like pH, hardness, conductance and TDS of the water samples underwent a change. On the other hand, the physical parameters like viscosity, density, were not much affected. These studies highlight the danger of dumping the discarded electronic components in river beds especially when the water flow is less and when the water is stagnated.
KEYWORDS:heavy metals;toxicity;surface water;leacheate;water quality
Download this article as:| Copy the following to cite this article: |
| Copy the following to cite this URL: |
Introduction
The electronic revolution is certainly a boon to mankind but at the same time, the use and throw culture and the greed of the human race to adopt new technologies have resulted in the generation of wastage of unwanted, discarded or broken electrical or electronic appliances, which is called e-waste. The rate at which e-waste mounts up has become a global concern. In the present scenario, the used electronic and electrical equipment reach the unorganized sectors where the recyclers perform dismantling, in informal recycling yards and dump the e waste in the land or discard the e-waste into water resources.1-5
The Central Pollution Control Board of India [CPCB] has projected that the country will generate more than 8 lakh tonnes of e- waste in 2012. According to the report, 65 cities in India generate more than 60% of the total e- waste. Tamil Nadu accounts for the second largest quantity of e-waste after Maharashtra, and is followed by Andra Pradesh, Utter Pradesh, West Bengal, Delhi, Karnataka, Gujarat, Madhya Pradesh and Punjab. Chennai ranks fourth after Mumbai, Delhi, and Bangalore among Indian cities that generate the most e-waste. 6 Toxic substances like lead, cadmium and mercury leach into the soil and ultimately pollute the ground water, if e-waste is dumped to the ground.
The polarity of water and hydrogen bonding enable water to dissolve, absorb, adsorb or suspend many different compounds. Thus, water can easily acquire contaminants from its surroundings. In the recent past e-waste has become the main source for ground water contamination.7-12Among the different types of contaminants affecting the water resources, heavy metals receive particular concern because of their strong toxicity even at low concentrations.13 Much attention has been given to heavy metal contamination in surface waters and ground water14-20. Extended studies and reviews on the negative impacts due to these hazardous elements on the environment and the human health are also reported 21-25. The first indication of such negative impacts is the change in quality of water when it is exposed to e-waste. Therefore this work is an attempt to study the change in the quality of water due to the leaching of heavy metals.
Materials and methods
Sample collection and preparation
Water samples were collected at random from two rivers Ganges and Indus in North India and two rivers Thamirabarani and Hanuman from South India. Discarded electronic circuit boards were collected from household waste and junkyard. The samples were heated to about 100oC in a furnace and crushed into minute particle using motor. The leachate solutions were prepared by taking 25g of the crushed material in 100mL of the different river water samples in a 250 mL beaker and allowing to stand for 7 days with occasional stirring. After 7 days, the suspension was filtered using 40 micron Whatman filter paper. The filtrate is called leachate.
Sample analysis
Determination of various physico-chemical parameters like conductivity and Total Dissolved Solids [TDS] were carried out following the procedures laid down by APHA26. Viscosity was determined by Oswald viscometer and ultrasonic velocity was determined using ultrasonic interferometer. The results are reported in Table 1 and Table 2
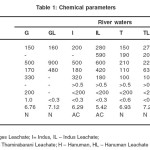 |
Table 1- Chemical parameters Click here to View table |
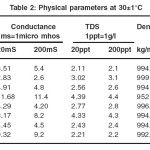 |
Table 2 – Physical parameters at 300C Click here to View table |
Quantitative analysis of the toxic heavy metals present in the leachate was carried out using Perkin Elmer Optima 5300DV ICP Optical Emission Spectrometer at the Sophisticated Analytical Instrument Facility [SAIF] , IIT, Madras and is reported in Table 3.The standards for water quality are given in Table 4.
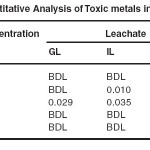 |
Table – 3 Quantitative Analysis of Toxic substances in the leachate Click here to View table |
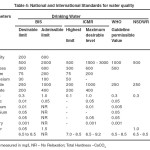 |
Table 4- Standards for water quality Click here to View table |
Results and Discussions
With the leaching of e- waste components in the water samples, the parameters like pH, hardness of water conductance and TDS have changed. The water sample from Indus River at the time of collecting the sample was more acidic in nature than the other samples and the leaching of e-waste components in Indus River water sample has made it still more acidic. For the other three rivers a slight increase in their pH values was observed. The hardness of water has increased in all the leachates. The conductance value of the water sample from Ganges has decreased to almost half in the leachate, while the values have almost increased to twice in the water samples from the other three rivers. The TDS values show an increase when the e-waste is leached in all the three rivers except that in Hanuman River. The physical parameters like density, viscosity and ultrasonic velocity did not vary much due to the leaching of e-waste components.
Table 5 highlights that though some of the toxic elements are Below the Detection Level, [BDL], there is a trace of Arsenic, Cadmium, Chromium, Lead and Mercury when the e-waste is allowed to leach in water.
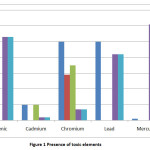 |
Figure 1 Presence of toxic elements Click here to View figure |
Maximum Contaminant Level (MCL) is the highest level of a contaminant allowed in a water system set at a numerical value with an adequate margin of safety to ensure no adverse effect on human health. The constituents of water for drinking purpose is different from that for irrigation use and certain standards are prescribed by various organizations like Bureau of Indian Standards (BIS), Indian Council of Medical Research (ICMR), World Health Organization (WHO) and Food and Agriculture Organization (FAO) of United Nations 20, 21 .The results were compared to the permissible limit of MCL specified by the Bureau of Indian standards (BIS) in Table 4. The five elements studied in this research namely Arsenic, Cadmium, Chromium, Lead and Mercury have MCLvalues of 0.05mg/L, 0.01 mg/L 0.05 mg/L 0.05mg/L and 0.001 mg/L respectively.
The average concentrations of the toxic elements evaluated by Atomic Absorption Spectrophotometer (AAS) in the surface water of the river near an industrial area in North India. They were Fe, Mn, Cu, Cr, Pb, Ni, Cd, As and Zn0.31, 1.08, 0.0076, 0.001, 0.0048, 0.0164, 0.00506 ppm, 0.2225 and 2.2 ppb respectively.22 Some of the toxic elements that were present in the Mithi river are Arsenic, Cadmium, Chromium, lead and mercury 23. River waters flow through large surface area through a long distance and the presence of heavy metals in such a situation may not occur at high concentrations. But if the discarded electronic components are thrown in river beds, when the water is scarce and flow of water is low, toxic metals can leach within a short period in stagnated water and make the water useless for drinking, irrigation and domestic purposes27.
Conclusion
This study has revealed that water samples collected at random from different geographical locations differ in their qualities. During a short contact period of 7 days, metals leach to different extent depending on the nature of water. The quality of water changes due to the heavy metal contamination leached from e waste.
Acknowledgement
Appreciation is expressed to the M.Sc. Physics students Ms. Lobsang, Ms.Shristi, Mr.Paul Daniel and Mr. Sriram for their effective involvement in the project work.
References
- Gaidajis G., Angelakoglou K. and Aktsoglou D., “E-waste: Environmental Problems and Current Management”, J Eng Sci and Technol Rev 3 (1) pp. 193-199 ,( 2010)
- “India faces e-waste challenge”,Times of India, 30 October 2009 [accessed July 2011]
- Ann Helwege. IR/GE 304 Environmentally Sustainable Development http://www.rsc.org/chemistryworld/News/2011/July/13071101.asp (2011.) [accessed Aug 2011]
- SnehalRebello. “City is an e-waste bin”, Hindustan Times, Mumbai, May 30,(2011).
- Toxics alert 3 August 2011 [accessed August 2011]
- Siddiqui F.Z. and Manuja S., “Environmentally Sound Management of E-waste: An Indian Perspective”, Env.Poll. Contr.J., 11(4), pp 21-30 (2008).
- VanloonG.W. and Duffy S.J., “The Hydrosphere” Environmental Chemistry: A Global Perspective, 2nd Edn. Oxford University Press, New York, pp. 197-211,( 2005).
- McMurry J. and Fay R.C.“Hydrogen, Oxygen and Water”, McMurry Fay Chemistry. K.P. Hamann, (Ed.). 4th Edn. Pearson Education, New Jersey, pp. 575-599, (2004).
- Mendie U. “The Nature of Water”, The Theory and Practice of Clean Water Production for Domestic and Industrial Use, Lacto-Medals Publishers, Lagos, pp.1-21, (2005).
- “Water for Pharmaceutical Use, Quality Assurance of Pharmaceuticals A Compendium of Guidelines and Related Materials.WHO, 2ndUpdated Edn. World Health Organisation, Geneva, (2007).
- Vodela J.K., Renden J.A., Lenz S.D., MchelHenney W.H. and Kemppainen B.W., “Drinking water contaminants”, Poult. Sci., pp.76, 1474-1492, (1997).
- Igwilo I.O., Afonne O.J., Maduabuchi U.J. and Orisakwe O.E., “Toxicologicalstudy of the Anam River in Otuocha, Anambra State, Nigeria”, Arch. Environ. Occup. Health, 61(5), pp.205-208, (2006).
- Marcovecchio J.E., Botte S.E. and Freije R.H., “Heavy Metals, Major Metals, Trace Elements”, Handbook of Water Analysis. L.M. Nollet, (Ed.). 2nd Edn.CRC Press, London, pp.275-311,( 2007).
- Mishra H.S. and Jha S.K., “Studies in River’s bottom Sediments in Swarnarekha River around Jamshedpur and Ghatsila for metallic pollution”, Curr. World Environ, 7(1), pp.63-167, (2012.).
- Acharyya S.K., Chakraborty P., Lahiri S., Raymahashay B.C., SaumyenGuha and AmitavaBhowmik. “Arsenic poisoning in the Ganges delta”, Nature, pp.401,545, ( 1999).
- Singh A.K., “Chemistry of arsenic in groundwater of Ganges–Brahmaputra river basin”, Curr. Sci., 91, (5), pp. 599-606, (2006.).
- HülyaKaradede-Akin, ErhanÜnlü. “Heavy Metal Concentrations in Water, Sediment, Fish and Some Benthic Organisms from Tigris River ,Turkey.”, Environ. Monit.Assess., 131(1-3) pp.323-337,( 2007).
- Jaleel Tariq, Ashraf M., Jaffar M. and Afzal M., “Pollution status of the Indus River, Pakistan, through heavy metal and macronutrient contents of fish, sediment and water,”Water Res.,30(6), pp.1337–1344,( 1996).
- DajkumarSahayam J., Chandrasekar N., KrishnaKumar S. and Victor Rajamanickam G., “Distribution of arsenic and mercury in subtropical coastal beachrock, Gulf of Mannar, India” , J. Earth Syst. Sci.,119(1),129–135,(2010).
- Mahfuza S., Sultana, Kulsum U., Shakila A. and Islam M.S., “ Toxic Metal Contamination on the River near Industrial Area of Dhaka”,Universal J Environ Res Technol, 2(2), pp.56-64 ,( 2012).
- Pravin U. Singare, Ravindra M. Mishra, ManishaTrivediP.,“Sediment Contamination Due to Toxic Heavy Metals in Mithi River of Mumbai,”Advances in Analytical Chemistry , 2(3), pp.14-24,( 2012).
- Reza R. and Singh G., “Heavy metal contamination and its indexing approach for river water”, Int. J. Environ. Sci. Technol., 7(4), pp.785-792, (2010).
- Jeffrey D. Weidenhamer. Circuit Board Analysis for Lead by Atomic Absorption Spectroscopy in a Course for Non-science Majors”, J.Chem.Educ. 84(7), 1165,( 2007).
- Amal K. Mitra ,AkhlaqueHaque, Manirul Islam and Bashar S.A.M. K. ,. “Lead Poisoning: An Alarming Public Health Problem in Bangladesh, Int. J. Environ. Res. Public Health,6, pp.84-95, (2009).
- Brett H., Robinson, “E-waste:An assessment of global production and environmental impacts”, Sci. Total Environ.,408 ,pp.183–191, (2009).
- APHA, Standard Methods for Examinatin of Water and Wastewater, 20th Ed., American Public Health Association, Washington,D.C. (1998).
- Bhadada R and Dharwal A.,E-Technologies Obsoletion- Environment Pollution Hazards and remedies,.Asian J Chem and Env Reser. 3(3), 84-91, (2010).

This work is licensed under a Creative Commons Attribution 4.0 International License.









