Spectrophotometric Study of Stability Constants of Cr(III), Ni(II) and Cu(II) Complexes with a Schiff’s Base in Different Solvents
Israel Leka Lere, Mamo Gebrezgiabher Beyene, Mulugeta Chekol and R.K.Upadhyay*
Department of Chemistry, N. C. S. College, Haramaya University, Ethiopia
DOI : http://dx.doi.org/10.13005/ojc/290336
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
Complexation of Cr(III), Ni(II) and Cu(II) with para-dimethylaminoanil of ortho-hydroxyphenylglyoxal Schiff’s base in methanol, ethanol and acetone solvents has been studied spectrophotometrically at room temperature (298K). The stoichiometry and stability of the complexes were determined using mole-ratio method. Stability data shows solvent-wise stability order as methanol > ethanol > acetone.
KEYWORDS:Free energy;Ortho-hydroxyacetophenone;para-dimethylaminoaniline;stability constant
Download this article as:| Copy the following to cite this article: Lere I. L, Beyene M. G, Chekol M, Upadhyay R. K. Spectrophotometric Study of Stability Constants of Cr(III), Ni(II) and Cu(II) Complexes with a Schiff’s Base in Different Solvents. Orient J Chem 2013;29(3). http://dx.doi.org/10.13005/ojc/290336 |
| Copy the following to cite this URL: Lere I. L, Beyene M. G, Chekol M, Upadhyay R. K. Spectrophotometric Study of Stability Constants of Cr(III), Ni(II) and Cu(II) Complexes with a Schiff’s Base in Different Solvents. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=333 |
INTRODUCTION
Although owing to versatile properties of Schiff’s bases they have multifarious roles in diverse fields, viz. industries1, analytical2,3, coordination4,5 and organic chemistry6,7, agriculture8, medical sciences9,10 etc. but ketoazomethines exhibiting novel ligation properties in forming heteropolynuclear11 and isomeric complexes12 and also adducts in unusual coordination numbers13,14 with transition metals attract high attention as ligands.
The effective coordination of ligands with metal ions may have significance in biological implications15. The chelation therapy for intoxication of heavy metals depends upon the chelating agent being able to reach the intercellular site where the heavy metals are firmly bound16. For a pretty long time, varieties of ligands have been used as antidote to combat metal poisoning. As Cr(III), Ni(II) and Cu(II), essential trace elements of mammals, usually form stable complexes with N, O and S donor ligands, a bidentate Schiff’s base, para-dimethylaminoanil of ortho-hydroxyphenylglyoxal (L), with N, O donor sites, therefore, has been used as ligand for spectrophotometric study of stability constants of complexes of Cr(III), Ni(II) and Cu(II) ions in different solvents (methanol, ethanol and acetone) and report in the present communication.
EXPERIMENTAL
Ortho-hydroxyacetophenone, para-dimethylaminoaniline, selenium dioxide and chlorides of Cr(III), Ni(II) and Cu(II) reagent grade chemicals of 97-98% purity obtained from CDH, Fluka and BDH were used in synthetic work without further purification. Solvents of analytical grade purity were used as supplied.
Spectrophotometric measurements were done on Shamdzu SP65 UV-Visible spectrophotometer in 200-800 nm range.
Synthesis of Ligand
Ortho-hydroxyphenylglyoxal, precursor of the ligand, was prepared16 by oxidation of ortho-hydroxyacetophenone with selenium dioxide in ethanol. The ligand was prepared by condensation of ortho-hydroxyphenylglyoxal and para-dimethylaminoaniline. On mixing equimolar quantities of the reactants (0.5 mol) in 100 ml of ether (predried with anhydrous Na2CO3) followed by stirring, precipitate17 of product was obtained, washed with dry ether and dried in air.
Preparation of Standard Stock Solutions
Equimolar (1×10-2M) stock solutions of ligand and metal chlorides were prepared by dissolving their requisite quantities in each of the solvents (methanol, ethanol and acetone) and diluting to 100 ml in volumetric flask.
Preparation of Working Solutions
Dilute solutions of the metal ions and ligand under study were obtained by appropriate dilution of stock solutions with their solvents.
Selection of Analytical Wavelength of the Complexes
For the selection of analytical wavelength Vosburgh and Cooper procedure was adopted18. A number of solutions are made by mixing different volumes of stock solutions of reactants in the same solvent. The absorbance were scanned between 200-800 nm at the difference of 10 nm to determine the λmax using UV-Visible spectrophotometer. The wavelength of maximum absorbance for each complex is noted in Table 1.
Procedure for Mole-Ratio Method
The procedure described by Nardo and Dawson19 was followed. 0.5 ml stock solution of each metal chloride in each solvent was pipetted into each of the sixteen test tubes and aliquot (0, 0.1, 0.2, 0.3, ———1.5 ml) of stock solution of ligand was added. In each test tube of metal-ligand mixture total volume was raised to 2 ml by adding the solvent (1.5, 1.4, 1.3, ——–0 ml). The reaction mixtures were shacked. The absorbance of each of the mixture was taken at λmax of the complexes in their solvents at room temperature (298K)
RESULTS AND DISCUSSION
Properties of Complexes
Ligand reacted with Ni(II), Cu(II) and Cr(III) to give orang red, orang yellow and greenish yellow complexes respectively in all the three solvents. Ligand showed λmax at 250 nm, 290 nm, 300 nm whereas Cr(III), Ni(II) and Cu(II) chlorides exhibit λmax at 515 nm, 510 nm and 415 nm in methanol, ethanol and acetone respectively. λmax of complexes are noted in Table 1. On heating the metal and ligand mixture solution (stoichiometric ratio) up to 600C unchanged colour intensity reveals independence of stability of complexes upon temperature.
Composition and Stability Constant of the Complexes
The stoichiometric ratio of the ligand to Cr(III), Ni(II) and Cu(II) in their complexes was determined by mole-ratio method at constant metal ion concentration (1×10-2M) and varying ligand concentrations (1×10-2M) and optical density was measured at the λmax of each complex in respective solvents. The intercepts of tangents on both arms of the graphs plotted between optical density and varying concentrations of ligand indicate 2:1, 1:1 and 2:1 ligand to metal interaction ratios in the complexes respectively in all the three solvents (Figures 1-3). The suggested metal ligand interactions are:
Cr3+ + 2 L → [Cr-L2]
Ni2+ + L → [Ni-L]
Cu2+ + 2L → [Cu-L2]
The stability constants of the complexes have been calculated by using the following equations:
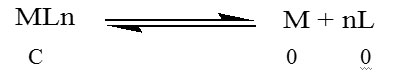
C (1- α) αC nαC
Where C is the total molar concentration of the complex assuming no dissociation, α is degree of dissociation and αC and nαC are equilibrium concentrations of the metal and ligand respectively.
The stability constant (Ks) = C (1-α)/αC.(nαC) n—– (i)
and α = (Em– Es)/ (Em) ————————————(ii)
Where Em is maximum extinction (Lmol-1cm-1) obtained from intercept of tangents on both arms of the curve and Es is extinction at equilibrium concentrations of complex and reactants obtained by intercept of mole ratio curve by the line parallel to y-axis through Em. The value of n is number of ligand to metal.
The value of Gibbs free energy (∆Go) of the complexation reactions is calculated by
– ∆G 0 = 2.303RT logks
The perusual of stability data (Table 1) reveals solvent-wise stability order for each of the complex as methanol > ethanol > acetone in accordance of polarity (dielectric constant) of solvents which indicates high covalent character of metal-ligand bond(s) in the title complexes. The negative free energy values indicate thermodynamic stability of complexes i.e independence of stability upon temperature.
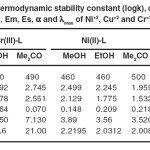 |
Table 1.The thermodynamic stability constant (logks), change in free energy (ΔGo), Em, Es, α and λmax of Ni+2, Cu+2 and Cr+3 complexes.: Click here to View table |
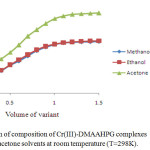 |
Figure 1. Determination of composition of Cr(III)-DMAAHPG complexes by mole ratio method in methanol, ethanol and acetone solvents at room temperature (T=298K). Click here to View figure |
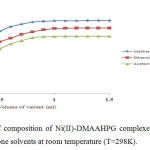 |
Figure 2. Determination of composition of Ni(II)-DMAAHPG complexes by mole ratio method in methanol, ethanol and acetone solvents at room temperature (T=298K). Click here to View figure |
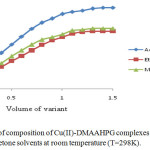 |
Figure 3. Determination of composition of Cu(II)-DMAAHPG complexes by mole ratio method in methanol, ethanol and acetone solvents at room temperature (T=298K). Click here to View figure |
CONCLUSION
1.The values of stability constants and negative sign of entropy changes of the complexes in the solvents used indicate that metal-ligand reactions are endothermic and complexes are stable at higher temperatures.
2.Solvent-wise stability order of complexes, methanol > ethanol > acetone, in accordance of polarity of solvents indicates high covalent character of metal-ligand bond(s).
3. Stability constant results suggest that ligand used is a good chelator agent for all the three metals.
REFERENCES
- Upadhayay R K and Agarwal N, Nat Acad Sci Lett., 1991, 14, 251.
- Upadhayay R K and Tiwari A P, J Indian Chem Soc., 1981, 58, 192.
- Bajpai A K and Upadhayay R K, Proc Nat Acad Sci Lett., 1987, 57A, 111.
- Upadhayay R K, Verma M and Madhu, J Indian Chem Soc., 1997, 74, 135.
- Upadhayay R K, J Indian Chem Soc., 1995, 72, 589.
- Upadhayay R K, Agarwal N and Gupta N, J Indian Chem Soc., 1993, 70, 537.
- Upadhayay R K, Megha, Upadhyay S and Jain S, E-Journal of Chem., 2009, 6, 254.
- Amico J J D, Patent U S., 1976, 3992192.
- Vats V, Upadhayay R K and Sharma P, E-Journal of Chem., 2010, 73, 1040.
- Cohen V I, Rist N and Duponchel C, J Pharm Sci., 1977, 66, 1332.
- Upadhayay R K, Verma M, Sharma M K and Madhu, J Indian Chem Soc., 1997, 74, 139.
- Upadhayay R K, Sharma V and Singh V P, J Lig Chromatogr., 1982, 5, 1141.
- Upadhayay R K, J Indian Chem Soc., 1997, 75, 535.
- Upadhayay R K, Bajpai A K, Rathore K, Transition Met Chem., 1985, 10, 24.
- Ajibola A O, Essential Medical Chemistry, 2nd Edn, Shaneson Jeresey, 1999, 2b.
- Klassen C D and Biliary, Drug Metal Rev., 1979, 5, 165.
- Agarwal N, PhD Thesis Meerut Univ Meerut (India), 1993
- Vosburgh W C and Cooper G R, J Amer Chem Soc., 1941, 63, 437.
- Nardo J V and Dawson J H, Inorg Chem Acta, 1986, 123, 9.

This work is licensed under a Creative Commons Attribution 4.0 International License.









