Albendazole Solubilization in Aqueous Solutions of Nicotinamide: Thermodynamics and Solute Solvent Interactions
Sushree Tripathy and Pravin Kumar Kar*
Dept of Chemistry, VSS Technical University, Burla, Orissa, India-768018
DOI : http://dx.doi.org/10.13005/ojc/290335
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
The present study deals with experiments so as to highlight the solute (drug albendazole) – solvent ( water) interactions and related thermodynamic modifications in presence of the hydrotropic agent nicotinamide at different temperatures T (= 298.15 to 313.15)K. Density and conductivity values of albendazole have been determined in water in (0.2, 0.4, 0.6, 0.8, 1 and 2) mol dm-3 aqueous solutions of nicotinamide at temperatures T(= 298.15, 303.15, 308.15 and 313.15)K, where as solubility was studied at 308.15. A concentration dependent solubility enhancement of albendazole was observed. The solubility data was treated to obtain the concentration dependent solubilization efficiency and the Gibbs free energy of transfer (∆G0tr ) of albendazole from pure water to the solvent systems. From the density values, the limiting partial molar volumes and expansibilities have been calculated. The limiting molar conductance (L0) and Arrhenius activation energy (Es) values have been calculated from the generated conductance values. The thermo physical parameters were discussed in terms of solute solvent interactions.
KEYWORDS:Albendazole;Nicotinamide;Solubility;Partial molar volumes;Arrhenius activation energy
Download this article as:| Copy the following to cite this article: Tripathy S, Kar P. K. Albendazole Solubilization in Aqueous Solutions of Nicotinamide: Thermodynamics and Solute Solvent Interactions. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290335 |
| Copy the following to cite this URL: Tripathy S, Kar P. K. Albendazole Solubilization in Aqueous Solutions of Nicotinamide: Thermodynamics and Solute Solvent Interactions. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=331 |
Introduction:
The poor aqueous solubility of therapeutically active chemical compounds i.e. drugs may limit their efficacy and utility. Solubility of drugs in aqueous media is an important factor that influences their dissolution rate and bioavailability after oral administration (1,2).There are several techniques and solubilizing agents that enhance the solubility of such drugs.
Amongst these techniques, hydrotropic solubilisation is considered as one of the safest methods of solubilisation. Aqueous solubilisation of insoluble drugs can be achieved by addition of hydrotropic agents (3). Nicotinamide (NIC) have been reported to enhance the aqueous solubilities of many drugs through intermolecular complex formation (4, 5, 6, and 7). The solubility enhancing effect of NIC has been attributed to its hydrotropic effects, where it is postulated to interact with solvent to enhance solubility (8). Another school of thought suggests stacking complexation as the mechanism of solubility enhancement of the drugs (9, 10). NIC enhanced the solubility of a poorly soluble drug albendazole (ALB) so to formulate the aqueous solution (11, 12, and 13). Many works highlighted the effect of NIC (14, 15) and hence improved solubility of the drug, but no detailed explanation is available relating to the improvement phenomenon in terms of solute-solvent interaction.
In this communication we aim to study the solute-solvent interaction and the related thermodynamics during the nicotinamide aided solubility enhancement of ALB.
It is well known that volumetric3 and conductometric (16, 17, and 18) studies of the solutions at definite and infinite dilution in a solvent system provide valuable information regarding the ion-ion and ion-solvent interactions. By examining the limiting apparent molar volume fv0, limiting molar expansibility , limiting molar conductance (L0) of solvent systems as a function of size, nature, temperature and composition of the solvent, it is possible to examine the parameters on solute-solvent interaction with the hope of obtaining a better understanding of interactions in solutions. The Gibbs free energy of transfer (∆G0tr ) of ABZ from pure water to the solvent systems and the Arrhenius activation energy (Es)values highlight the thermodynamics involved during the solubility enhancement.
The present study deals with experiments so as to highlight the solute (drug ALB) – solvent (water) interactions and related modifications in case of the presence of NIC at different temperatures T(=298.15 to 313.15)K.
Experimentals:
Materials
Nicotinamide (NIC) was of Analytical Reagent grade and kept over anhydrous CaCl2 in a vacuum desiccator until required. Albendazole(ALB) was a gift sample (procured from M/S Alkem Laboratory, Mumbai, India) and used as such. The solutions were prepared freshly by mass using a Metler balance with a precision of ±0.01mg in doubly distilled deionized and degassed water, and conversion of molality to molarity was done by standard expression (3).
Phase Solubility studies
Different concentrations (0.08 to 0.98 M) of NIC was prepared in double distilled water using 100 ml conical flask, and excess amount of drug, ALB was added to each flask containing the required molar solutions of NIC. All the conical flasks were put in a mechanical shaker (Remi-RSB-12) maintained at the temperature of 308.15 K within an accuracy of ± 0.02K and shaken for a maximum period of 72 hours (19). After 72 hours, 10ml aliquot from each flask was withdrawn, filtered with a 0.5 micron filter paper and analyzed at 291 nm by UV double beam spectro-photometer (model Hitachi U3010) for determining the aqueous solubility of ALB in different solutions of nicotinamide at temperature 308.15K.
Measurement of density and conductivity
An Anton Paar digital densimeter (Model DMA 60/602) was used to measure the densities of the solutions. An efficient constant temperature bath with stability within ±0.02K was used to control the temperature of water around the densimeter cell. The densimeter was calibrated by using pure water and dry air. All the density measurements of the solutions were made relative to pure water.The densities have precision better than ±10 -3 kg m-3.
A conductivity meter with accuracy of ±0.5 % and a conductivity cell ( Systronics Model –306) were used for the measurement of conductivity of each sample. The conductance cell was equipped with a water circulating jacket, and the temperature was controlled within ±0.02K with a water thermostat. The cell constant is 1.01cm -1 which was calculated by repeated measurements of KCl solutions. For each sample six datapoints were considered to calculate the average, which was further used to calculate the equivalent conductance for the samples. All data were corrected with specific conductivity of pure water at the experimental temperatures.
Results and discussion
Phase solubility study.
The results of the solubility measurements of ALB in different test solutions containing nicotinamide are shown in Table-1. It is seen that the solubility of ALB increased significantly in presence of NIC than that in pure water. The solubility enhancement was observed to be concentration dependent as evidenced by a 102.63 fold increase in ALB solubility in 0.98 M solutions containing NIC as compared to that of in water. This observation contradicts to the earlier report (12) that mentions only a 17 fold increment in ALB solubility in 2.0M NIC solutions. The huge differences may be attributed to (i) the measurement wavelength maximum of 308 nm as compared to that of 291nm in our case and (ii) the duration of mechanical shaking of 12 hours against 72 hours in our case.
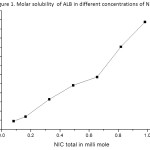 |
Figure 1. Molar solubility of ALB in different concentrations of NIC. Click here to View figure |
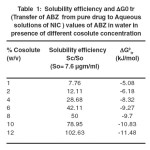 |
Table 1. Solubility efficiency and ∆G0 tr ( Transfer of ABZ from pure drug to Aqueous solutions of NIC )values of ABZ in water in presence of different cosolute concentration. Click here to View table |
Further the solubility increment of ALB (Figure-1) was not a linear function of NIC concentration which suggests the formation of higher order complexes.
The Gibbs free energy of transfer (∆G0tr) of ALB from pure water to aqueous solutions of NIC was calculated using the equation in the form of
(∆G0tr)= – 2.303RT Log [Sc/S0] (1)
Where [Sc/S0] is the ratio of molar solubility of ALB in aqueous NIC solutions to that of pure water. The Gibbs free energy change is an indication of the process of ALB transfer from pure water to aqueous NIC solutions, that otherwise reflects the extent of favourability in regard to the solubility enhancement phenomena. The values of ∆G0tr are shown in Table 1. The negative ∆G0tr values indicate favourable conditions. For the different aqueous NIC solutions the ∆G0tr values are all negative and are observed to decrease with an increase in ARG molar concentration. This observation can be explained by the fact that solution environment becomes more favourable with increase in NIC presence so to enhance the accommodation of ALB in water.
Conductivity
The molar conductance data (Λ) with different concentrations of pure NIC and that of the ALB solubilised different NIC solutions are listed in Table 2 and Table 3, respectively. The limiting molar conductivity (Λ₀) of the systems was obtained by least squares fitting the experimental data to the expression (20)below:
Λ = Λ₀ – A√c / (1+B √c), (2)
Where Λ₀, A and B are fitting parameters, c is the concentration. The obtained limiting molar conductivities of the systems are summarized in Table 2 and Table 3.
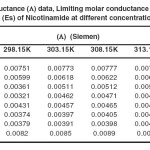 |
Table 2 Conductance (Λ) data, Limiting molar conductance (Λ₀) data and Activation energy (Es) of Nicotinamide at different concentration & temperature. Click here to View table |
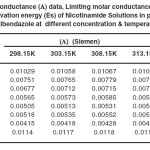 |
Table 3. Conductance (Λ) data, Limiting molar conductance (Λ₀) data and Activation energy (Es) of Nicotinamide Solutions in presence of Albendazole at different concentration & temperature. Click here to View table |
It can be seen from Table 2 and 3, that the values of Λ both in NIC solutions and ALB solubilised NIC solutions decrease with increasing NIC content whereas increase, as the temperature increases. This can be ascribed to the fact that (i) an increase in NIC concentration enhances the microscopic viscosity of the medium because of increased frictional coefficient of the medium there by retarding the mobility of the polymeric units in the solution, (ii) higher solvation leads to an increase in the hydrodynamic radii of the NIC unit thereby decreasing their mobility and (iii) with increase in temperature the increased thermal energy results a higher mobility because of the greater bond breaking and variation in vibrational, rotational and translational energy of the solute (21).
As observed in Table 2 and 3, the values of Λ₀ in NIC solutions at different temperatures are lower as compared with those ALB solubilised NIC solutions systems. The Λ₀ values are found to increase with temperature in both the systems. The higher values of Λ₀ in ALB solubilised NIC systems than that of its counterpart without ALB can be attributed to the formation of the solute – cosolute associations there by reducing their overall number. This phenomena reduces the microscopic viscosity of the system as well as the net resistance to the mobility.
Since the conductance measurements of an ion depend upon mobility(22), it is quite reasonable to treat the conductance data similar to the one employed for the rate process taking place with the change of temperature, i.e.:
Λ₀ = A e-Es/RT (2)
Where A is the frequency factor, R is the gas constant and Es is the Arrhenius activation energy of the transport process.
From the plot of log Λ₀ vs. 1/T, the Es values have been computed from the slope (=-Es/2.303R) and had been recorded in Table 2 and 3. It can be seen that the values of Es are positive both for individual solutions and the system of NIC, which indicates to the fact that NIC dissolution is a favourable process. The similar observations were made in case of the ALB solubilised NIC systems, which suggest the process of solubilisation to be favourable. The results of the phase solubility study and thermo physical observations are complimenting each other.
Density
The results of the density measurements are given in Table 4 along with the values of limiting apparent molar volume (Φv0) and limiting apparent molar expansibility (ΦE0) of the systems containing different concentrations of pure NIC and that of the ALB solubilised different NIC solutions in water at T= (298.15, 303.15, 308.15, 313.15)K. The values of Φv0 and ΦE0 have been determined from the intercept of the plot of Fv vs c1/2 and of FE vs c1/2, respectively, which are in turn determined from the experimentally measured densities as follows:
Φv=1000(cd0)-1(d0-d) +M2/d0 (3)
and
ΦE=αΦv0+ (α-α0) 1000c-1 (4)
Where c is the molar concentration of the solute, d0 is the density of pure water, d is the density of the solution, M2 is the molecular mass of the solute and a0 and a are the coefficients of expansion of the solvent and solution (with or without drug), respectively, and determined by means of the relation available in the literature (23).
The Φv and ΦE showed linear dependence with square root of concentration and were found to obey the linear equations,
Φv=Φv0+Svc1/2 (5)
and
ΦE=ΦE0+SEc-1 (6)
The values of SV and SE obtained from the slopes of the corresponding plots are also given in Table 4.
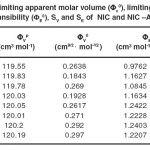 |
Table 4. Limiting apparent molar volume (Φv0), limiting apparent molar expansibility (ΦE0), SV and SE of NIC and NIC –ALB System. Click here to View table |
A perusal of Table 4 shows that the values of Φv0 are positive for both the systems at the experimental temperatures. Since the Φv0 is a measure of ion-solvent interaction, the positive values of Φv0 indicate strong solute-solvent interactions. However if compared between the two, ALB solubilised NIC system showed stronger interactions than that of the NIC at lower temperatures (298.15 and 303.15) as supported by higher values of Φv0, but at the higher temperatures of 308.15 and 313.15K the reverse is the case. The results indicate that the solute solvent interactions in both the systems increase with increase in temperature. The presence of solute-solvent interaction amongst the molecules, promotes the structure making effects of the solute in water.
As observed, the SV values are positive for both the systems and at all experimental temperatures. The positive value indicates a strong solute solvent interaction. The SV values are higher in case of ALB solubilised NIC systems at lower temperatures (298.15 and 303.15) than that of NIC systems where as at the higher temperatures of 308.15 and 313.15K the reverse is the case. This point to the fact that the solvent structure is too much enhanced in the lower temperature, where as it is not too much enhanced at higher temperatures (3).
The values of ΦE0 for all the systems at all temperatures are small but positive and increase with temperature except that of 313.15K where it decreases. However, the values of ΦE0 in the in case of ALB solubilised NIC systems as compared with that of NIC systems indicated similar trend as that of the SV values. These observations indicated the presence of caging or packing effect (22) supporting to the earlier contention that the solvent structure is too much enhanced only at experimental temperatures of 308.15 and 313.15 K.
Conclusion
In this study the NIC aided aqueous solubilization of ALB has been revisited. We found 102.63 fold increase in solubility of ALB as compared to that of its intrinsic solubility. The ∆G0tr values have been calculated from the solubility data and its negative value indicated the favourable process of ALB transfer from pure water to aqueous NIC solutions.
To understand the related solute solvent interactions and involved thermodynamics during the solubility enhancement of ALB in presence of NIC, the different strengths of NIC solutions in absence and saturated presence of ALB have been subjected to conductometric and volumetric investigations hence the conductivities and densities have been determined at the temperature range between 298.15 and 313.15 K.
The limiting molar conductivity (Λ₀) of NIC system was lower than that of the ALB solubilised NIC systems. This observation suggests the formation of a solute-cosolute association that results a decrease in microscopic viscosity of the medium hence enhancing their mobility. In both systems the Λ₀ values increase regularly with increase in temperature. The Arrhenius activation energy of the transport process was found to be positive in both systems of NIC and ALB solubilised NIC indicating the favourable solute solvent interaction.
The positive values of Φv0 and ΦE0 indicated a temperature dependent strong solute solvent interactions and a possible packing effect involving structure making. The positive value of Sv indicated a strong solute-solute interaction which is further supported by solute cosolute association.
References:
- Sanghvi R, Evans D, Yalkowsky S H, Int. J. Pharm., 336, 35-41(2007).
- Kallinteri P, Antimisiaris SG, Int. J. Pharm., 221, 219-226 (2001).
- Solanki C S, Tripathy S, Tripathy M K, and Dash U N, E-J Chem., 7(S1), S223-230(2010).
- Ahuja N, Katare O P, Singh B, Eur. J. Pharm. and Biopharm., 65, 26-38 (2007).
- Bley H, Fussnegger B, Bodmeier R,Int. J. Pharm.,doi:10.1016/j.ijpharm.2010.01.039 (2010).
- Wang L, Xuehua Jiang, Weijuan X, Chenrui, Int. J. Pharm., 341, 58-67(2007).
- Abdul Rassol A, Hussain A, Dittern L W, J. Pharm. Sci., 80, 387-393 (1991)
- Coffman R E, Kild Sig D O, J. Pharm. Sci., 85, 951-954 (1996).
- Suzuki H, Sunada H, Chem. Pharm. Bull. 46, 125-130 (1998).
- Dasilva RC, Spitzer M, Dasilva LHM, Low W, Thermochim. Acta, 328, 161-167 (1999).
- Go M, Lim L, Eur. J. Pharm. Sci., 10, 17-28 (2000).
- Balaji NJ, Kulkarni PK, Prabhuling VR, Indian J. Pharm. Educ. Res., 41(2), 150-154 (2007)
- Tripathy S, Kar P K, Majeed A B A, Int. J. Pharm. Bio. Sci., 4(1), 306-319 (2013).
- Agrawal S, Pancholi S S, Jain N K and Agrawal G P, Int J. Pharm., 2004, 274,149.
- Poochkian G D and Gradock J C., J.Pharm.Sci, 1974, 68, 728.
- Wang S, Jacquemin J, Husson P, Hardacre C, Costagomes M F, J. Chem. Therm., 2009, 41, 1206.
- Solanki C, Mishra P, Talari MK, Tripathy MK, Dash UN, E-J Chem., 9(1), 21-26 (2012).
- Tripathy S, Tripathy MK, Kar P K, Majeed A B A, Chem. Sci. Trans., 2(1), 208- 212 (2013).
- Aggarwal A K, Jain S, Chem Pharm Bull., 59(5), 629-638 (2011).
- Harned H S and Owen B B, The Physical Chemistry of Electrolyte Solutions; 3rd Ed.; Reinhold, New York, 1958; pp. 358-376.
- Dash U N, Mohapatro J and Lal B, J. Mol. Liq. 2006, 124, 13.
- Coetzee J F and Ritchi D, Solute solvent interactions; Marcell Dekkar: New York and Basel, 1976.
- Millero F J, Structure and transport properties in water and aqueous solutions; Edited by Horne, R.A., Wiley Interscience , New York, 1971, Chapter 15.

This work is licensed under a Creative Commons Attribution 4.0 International License.









