Solubility of Betulinic Acid in Microemulsion System: (Part 2)
Ahmad Zaidi B. Ismail, Nur Nadiah Abdul Rashid and Faujan B. H. Ahmad
Department of Chemistry, Faculty of Science, University Putra Malaysia - 43400 Serdang, Selangor (Malaysia).
Microemulsion has been reported to be an alternative carrier for poor solubility of the compounds in aqueous media. Microemulsion is a stable isotropic liquid mixture of oil, water, surfactant, with or without the combination of a co-surfactant. In this work, the microemulsion was designed by constructing a ternary phase diagram using sodium dodecyl sulphate (SDS) as the surfactant, water as the aqueous media and the carboxylic acids and esters as the co-surfactant. After designing the microemulsion, and constructing the phase diagram, the known isotropic regions were reformulated. Betulinic acid was then solubilized in the isotropic region. For the system containing carboxylic acid, the acetic acid system offered the largest isotropic region and it was found that the microemulsion system obtained was suitable for dissolving betulinic acid. The concentration of betulinic acid dissolved in this system was directly proportional to the percentage of surfactant but inversely proportional to the water percentage.
KEYWORDS:Betulinic acid; Microemulsion system
Download this article as:| Copy the following to cite this article: Ismail A. Z. B, Rashid N. N. A, Ahmad F. B. H. Solubility of Betulinic Acid in Microemulsion System: (Part 2). Orient J Chem 2011;27(3). |
| Copy the following to cite this URL: Ismail A. Z. B, Rashid N. N. A, Ahmad F. B. H. Solubility of Betulinic Acid in Microemulsion System: (Part 2). Orient J Chem 2011;27(3). Available from: http://www.orientjchem.org/?p=24417 |
Introduction
Betulinic acid, (3β)-3-hydroxy-lup-20(29)-en-28-oic acid (Figure 1), is a naturally occurring pentacyclic lupane-type triterpenoid that possess multiple pharmacological activities including inhibition of human immunodeficiency virus, antibacterial, antimalarial, anti-inflammatory, anthelmintic, antioxidant and anticancer properties.1,2 However, medical uses of betulinic acid in the pharmaceutical industry are limited owing to its insolubility in water (0.02 mg mL–1), which causes difficulty in preparing injectable formulations for biological assays and decreases bioavailability in the organism.
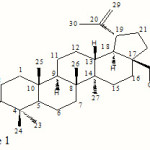 |
Figure 1 Click here to View figure |
Microemulsion is a stable isotropic liquid mixture of oil, water, surfactant, frequently in combination with a co surfactant. In 2006 Gosh et al., 3 has stated that microemulsion are homogeneous, transparent, thermodynamically stable dispersion of water and oil, stabilized by a surfactant usually in combination with a co surfactant.Co-surfactants are weakly amphiphilic molecules, which are assumed to concentrate in the surfactant layer of the aggregates formed by the primary surfactant.It was noted that due to their amphiphilic character, co-surfactant alone does not form aggregates, but they strongly support the aggregation of the primary surfactant.4
The application of this microemulsion is due to its own properties of an ultra low interfacial tension. This has made the microemulsions are suitable solvent for hydrophobic organic compound and inorganic salts. Thus much attention has been studied to the application of microemulsions as drug delivery systems, since microemulsions are thermodynamically stable and are formed spontaneously by simple mixing of the various components-surfactant- co-surfactant.5-8
Thus, in relation our early work on the solubilization of betulinic acid in microemulsion system,9 we know report our results on the solubility of betulinic acid in microemulsion system containing sodium dodecyl sulphate (SDS) as the surfactant, water as the aqueous media, and the carboxylic acids or esters as the co-surfactant .
Materials and Methods
Materials
Betulinic acid was donated by Prof. Dr. Faujan B H Ahmad. The surfactant, Sodium Dodecyl Sulfate (SDS) was obtained from BDH (Laboratory Grade), acetic acids and esters were obtained from Fluka Chemical and distilled water was used for the formulation.
Methods
Construction of phase diagram
Three ternary phase diagrams of water/carboxylic acid/surfactant for water/acetic acid/SDS; water/caproic acid/SDS; and water/oleic acid/SDS systems were first contructed. Then, four ternary phase diagrams of water/ester/surfactant for water/methyl acetate/SDS; water/ethyl acetate/SDS; water/n-amyl acetate/SDS; and water/isoamyl acetate/SDS systems were constructed. The surfactant and ester or carboxylic acid were weighted and mixed together in the ratio of 0/100 to 100/0 in different test tubes. The total weigh of the mixture was 0.5g. Addition of water was based on stepwise addition ranging from 5% to 95%. Every stepwise addition was followed with centrifugation at 5000 rpm to determine the phase formed whether the mixture is one-phase, two-phases or multiphase. All ternary phase diagrams were prepared at 300C and under low-emulsification methods. The compositions are expressed in wt. % ratio between components.
Study the solubility of betulinic acid in the microemulsion system.
After constructing the phase diagram and determine the system that has the largest isotropic region, betulinic acid was added into the microemulsion sample of the isotropic region. From the phase diagrams, two of the largest isotropic region was chosen. Then from the phase diagrams region are choose for different ratio and the betulinic acid was solubilize in the chosen point at different water percentage.
Results and Discussions
The ternary phase diagrams of water/carboxylic acids/SDS microemulsion system
The phase diagrams of the water/acetic acid/SDS; water/caproic acid/SDS; and water/oleic acid/SDS systems, at 300C, are shown in Fig. 2(i), 1(ii) and 1(iii) respectively. Based on Fig. 2, it came to a conclusion that the water/acetic acid/SDS system (Fig. 2(i)) composed the largest clear isotropic region. The shaded area shows the one-phase clear isotropic region while the unshaded area indicated the multiphase region. Fig. 2(ii) which is for the water/caproic acid/SDS system also composed clear isotropic region but the area was smaller if compared to water/acetic acid/SDS system. For water/oleic acid/SDS system (Fig. 2(iii)), it composed one-phase region but it was not a clear region. It gave a result of milky homogenous region. This observation may due to the size of hydrocarbon tail of the carboxylic acids. The longer hydrocarbon tails somehow restrain the formulation of the microemulsion.
 |
Figure 2: Comparison of three different phase diagrams of water/carboxylic acids/SDS microemulsion system |
The ternary phase diagrams of water/esters/SDS microemulsion system
The phase diagrams of water/methyl acetate/SDS; water/ethyl acetate/SDS; water/n-amyl acetate/SDS; and water/isoamyl acetate/SDS systems, at 300C, are shown in Fig. 3(i)-(iv), respectively. Water/methyl acetate/SDS system composed the largest isotropic region, followed by water/ethyl acetate/SDS system and water/n-amyl acetate/SDS system. The water/ethyl acetate/SDS system formed a smaller isotropic region because ethyl acetate has a longer hydrocarbon tails, and again it may somehow restrained the formation of a large isotropic region as in the carboxylic acids system as described above. Water/n-amyl acetate/SDS system formed a narrow area of isotropic and it is located almost at the edge of the phase diagram. This happened my due to the presence of a much longer hydrophobic group in the molecular structure of amyl acetate which also known as pentyl acetate.
As for the water/isoamyl/SDS system, it is shown that the ternary phase diagram composed the smallest isotropic region. This happened not only due to the long chain of hydrocarbon but also may due to the branched structure. The existence branch methyl group in isoamyl acetate may cause the isoamyl acetate slightly hindered to form aggregates and to be oriented closer to each other and the surfactant molecule. This can be called as stearic hindrance and caused the microemulsion difficult to be formed at higher ratio of isoamyl acetate. In this connection, the influence of hydrophobe branching in partitioning of ethoxylated alkylphenol surfactants in microemulsion-oil-water systems was noted in the literature10. Interesting to note that in all these ternary diagrams for all four types of esters used, the isotropic regions were obtained at high percentage of water.
 |
Figure 3: Comparison of four phase diagrams for water/esters/SDS microemulsion system |
Solubility of betulinic acid in H2O/carboxylic acids/SDS microemulsion system
The system with the largest isotropic region was chosen from the various carboxylic acids system. As described above, acetic acid system was observed has the largest microemulsion region in the phase diagram. The microemulsion was reformulated at a different ratio of SDS to acetic acid and different water percentage starting at 40% up to 80% of water. Betulinic acid was solubilized in the microemulsion and the maximum amount of betulinic acid dissolved in each point was determined.
It was found that the maximum amount of betulinic acid dissolved was at 0.95 mg for 1.0 g of reformulated microemulsion at the 40% of water and 30:70 ratio of SDS to acetic acid. It was observed that the weights of betulinic acid dissolved were inversely proportional to the water percentage. The solubilization was observed to decrease with the increment of percentage of water in contrast it was increase with the increase of surfactant ratio. These suggest that microemulsion can act as the alternative solvent for poorly aqueous soluble compound. The surfactant help in lowering the interfacial tension by forming aggregates called micelles. The observation is shown in Figure 4(i) and (ii), respectively.
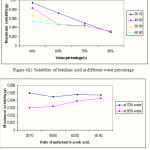 |
Figure 4 Click here to View figure |
Solubility of betulinic acid in H2O/esters/SDS microemulsion system
The microemulsion was reformulated at different water percentage and different ratio of methyl acetate and SDS. The maximum amount of betulinic acid soluble in the microemulsion was 0.35 mg for 1.0 g of reformulated microemulsion at 40% of water and 30:70 of SDS to methyl acetate ratio. The solubility of betulinic acid in this ester system seems much lower as compared to its solubility in acetic acid/SDS/H2O system as observed in above system.
Again, the amount of betulinic acid soluble in the microemulsion system decreasing with the increasing of water percentage as expected. This is my due to the hydrophobicity characteristic of betulinic acid itself. With the increase of surfactant ratio, the solubility of betulinic acid was observed also to be increase. The solubility of betulinic acid in the ester/SDS/H2O microemulsion system is shown in Figure 5(i), and 5(ii), respectively.
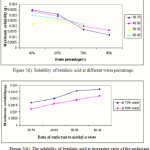 |
Figure 5 Click here to View figure |
Conclusion
Our observation suggested that microemulsion could be used as the alternative carrier for betulinic acid. The system contain water/acetic acid/sodium dodecyl sulphate, which was offered the largest isotropic region in the ternary phase diagram afford to dissolved up to 0.95 mg of betulinic acid, at 30:70 ratio of SDS to acetic acid and 40% of water in 1.0 gm of microemulsion. While the system using water/methyl acetate/sodium dodecyl sulphate only able to soluble betulinic acid up to 0.35 mg at 30:70 ratio of SDS to methyl acetate and 40% of water. Our present results suggested that betulinic acid is less soluble in microemolsion containing single surfactant as compared if the system containing a mix- surfactant, as reported in our early results.9
In both system either using acetic acid or methyl acetate as co-surfactant, it was observed that the solubility of betulinic acid was inversely proportional to the water percentage.
References
- Yogeeswari P, and Sriram D. 2005. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 12: 657 – 666.
- Faujan N. H., Alitheen N. B., Ye S. K., Ali A. M., Muhajir A. H., and Ahmad F. B. H.(2010): Cytotoxic effect of betulinic acid and betulinic acid acetate isolated from Melaleuca cajuput on human myeloid leukemia (HL-60) cell line. African Journal of Biotechnology. 9(38), 6387-6396.
- Ghosh P.K., Majithiya Rita J., Manish L.U., and Rayasa S.R. M. (2006): Design and Development of Microemulsion Drug Delivery System of Acyclovir for Improvement of Oral Bioavailability.AAPS Pharm Sci Tech. 7(3) Article 77 E1–E6.
- Naresh C., Henry B., Lauriane F., Scanu F., Siperstein R., and Keith E.G. (2005): Cosurfactant and cosolvent effects on surfactant self-assembly in supercritical carbon dioxide. The Journal of Chemical Physics. 122, 094710. 1-11.
- Anna K. , Abraham A., and Nissim G.(2007): Improved solubilization of carbamazepine and structural transitions in nonionic microemulsion upon aqueous phase dilution. Journal of Colloid and Interface Science. 315. 637–647.
- Aviram S. and Abraham A. (2006): Microemulsion as carriers for drugs and nutraceuticals. Advances in Colloid and Interface Science. 128–130. 47–64.
- Indranil N., Mohammad B. and Hemant J. (2003): Study of IsopropylMyristate Microemulsion systems Containing Cyclodextrin to Improve the Solubility of 2 Model Hydrophobic Drugs. AAPS Pharm Sci Tech. 4 (1). 1-9.
- Hiroshi A., Mikio T., and Masahiro H. (2005): The novel formulationdesign of O/W microemulsion for improving the gastrointestinal absorption of poorly water soluble compounds. International Journal of Pharmaceutics. 305, 61–74.
- W.M. Wan Rusmawati, F.B.H. Ahmad, K. Anuar, and S. Hamdan (2001): Part 1: Solubility of Betulinic Acid in Microemulsion System of Methyl Acetate/Tween 80/ Brij 30/ H20. Oriental J. of Chemistry. 16(3), 393-398.
- Nelson M. R. E., Anton A. G. and Jean Lachaise Jean-Louis Salager (1998): Partitioning of ethoxylated alkylphenol surfactants in microemulsion-oil-water systems. Part II: influence of hydrophobe branching. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 131, 45 49.

This work is licensed under a Creative Commons Attribution 4.0 International License.









