Solid Phase Extraction of Ultra Traces Copper (II) Using Octadecyl Silica Membrane Disks Modified by a New Schiff Base Prior to Determination by Flame Atomic Absorption
Mohammad Kazem Rofouei1, Hadis Ferdowsi Sh1,Mahmoodpayehghadr2* And Amir Mehdi Fat’Hi3
1Faculty of Chemistry, Tarbiat Moalem University, Tehran, Iran.
2Payame Noor University, Karaj, Iran.
3Department of Chemistry, Faculty of Science, Zanjan University, Zanjan, Iran.
A simple, reliable and rapid method for pre-concentration and determination of copper using octadecyl silica membrane disk modified by a recently synthesized Schiff base (N,Nbis(2-hydroxy-4 methoxybenzaldehyde)-2,6-diaminopyridine) and flame atomic absorption spectrometry is presented. Various parameters including pH of sample solution, flow rates, the amount of ligand and type of stripping reagent were optimized. Under optimum experimental conditions, the breakthrough volume is greater than 2000 ml with an enrichment factor of more than 400 and detection limit of 5.4 ng.L-1. The capacity of the membrane disks modified by 5 mg of the ligand was found to be 552.53 μg of copper. The effects of various cationic interferences on percent recovery of copper ion were studied. The method was successfully applied for the determination of copper ion in different water samples.
KEYWORDS:Copper (II); SPE, Octadecyl silica disks; Schiff base; AAS.
Download this article as:| Copy the following to cite this article: Rofouei M. K , Ferdowsi S. H, Payehghadr H, Fathi A. M. Solid Phase Extraction of Ultra Traces Copper (II) Using Octadecyl Silica Membrane Disks Modified by a New Schiff Base Prior to Determination by Flame Atomic Absorption. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Rofouei M. K , Ferdowsi S. H, Payehghadr H, Fathi A. M. Solid Phase Extraction of Ultra Traces Copper (II) Using Octadecyl Silica Membrane Disks Modified by a New Schiff Base Prior to Determination by Flame Atomic Absorption. Available from: http://www.orientjchem.org/?p=24644 |
Introduction
Copper are widespread anthropogenic pollutant of environment and the determination of this metal ion is an actual problem. The determination of copper is usually carried out by flame1-3 and graphite4-5 atomic absorption spectrometry (AAS), as well as spectrophotometry6-7, chemiluminescence8 and electrothermal methods9-10. However, due to presence of copper in low levels in environmental samples and the matrix effects, different separation and pre-concentration techniques such as liquid-liquid extraction11, precipitation12, ion exchange13, solid phase extraction14-15 and membrane filtration16 improve the analytical detection limit, increase the sensitivity by several orders of magnitude, enhance the accuracy of the results and facilitate the calibration. Among these techniques, solid phase extraction is preferred by many researchers on account of the fast, simple and higher pre-concentration factor, rapid phase separation, time and cost saving17-18. A number of supports have been widely used for the pre-concentration and separation of trace metal ions from various matrices. Among the absorbents, silica with chemically bonded alkyl chains such as octadecyl bonded silica (C18), modified by the use of suitable ligands has been an excellent used extractor of metal ions19-23.
The Schiff’s bases as poly dentate ligands are known to form very stable complexes with transition metal ions24-26. In this work, a newly synthesized ligand N,N-bis(2-hydroxy-4-methoxy benzaldehyde)-2,6-diamino pyridine (HMD)(Fig. 1) is studied as a disk modifier for Cu(II). Also, we report on extraction and pre-concentration of copper (II) from water samples and determination by atomic absorption spectrometry.
 |
Figure 1: Chemical structure of HMD Click here to View figure |
Experimental
Instrumentation
A Varian (220AA) flame atomic absorption spectrometer (air-acetylene flame) was used for determination of copper ions. A copper hollow cathode lamp was used as light source operated at 4 mA. The wavelength was set at 324.7 nm resonance line and the spectral band pass at 0.2nm. Crison GLP 22 pH meter was used to measure pH values.
Chemicals and Reagents
All chemicals used in this work, were purchased from Merck and were analytical reagent grade. Deionized double distilled water was used throughout. The stock standard solution of Cu2+ was prepared by dissolving 10 mg of Cu(NO3)2 in the least amount of 0.01 M HNO3 and diluting to 100 ml in a calibrated volumetric flask with water. Working solutions were prepared by appropriate dilutions of the stock solution. 0.1 M acetate, phosphate and formate buffer solutions were used for the pH range 4-6, 2, 7, 8 and 3, respectively.
Sample Extraction
Extraction were performed with 47 mm diameter × 0.5 mm thickness Empore membrane disks containing octadecyl – bonded silica (8 μm particle, 60 nm pore size, 3M company). The disks were used in conjunction with a with a standard Millipore 47 mm filtration apparatus. The membrane disk was placed in the filtration apparatus and it was washed with 20 ml methanol by applying a slight vacuum. With a thin layer of methanol remaining on the disk, it was washed with 10 ml of deionized water and it was then dried under vacuum for 5 min. It is important to also note that the surface of the disk did not let be dried until the extraction was completed.
Finally, a solution of 5 mg HMD in 3 ml DMSO was in to the disk and draws slowly through the disk by applying a slight vacuum. The passed solution was collected in a test tube. Then water was added drop-by-drop to the test tube until the formation of a suspension began, and the resulting suspension was again introduced on to the disk and passed through the disk slowly. The filtration step was repeated until the passed solution was completely clear. The membrane disk modified by HMD is now ready for sample extraction.Then a 100 ml sample solution containing 10 μg copper, adjusted to a pH 7.0 was passed through the disk. A 23 mm × 200 mm test tube was then placed under the extraction funnel. Copper extracted on disk was then eluted with 0.07 M HNO3. The copper content was then determined by FAAS.
Results and Discussion
Effect of Aqueous Phase Ph
HMD is an O2-N2 donating Schiff’s base which is insoluble in water at natural pH. Most chelating ligands (such as Schiff bases) are conjugate bases of weak acid groups and accordingly, have a very strong affinity for hydrogen ions. The pH therefore, will be a very important factor in the separation of metal ions by chelating, because it will determine the values of the conditional stability constants of the metal complexes on the surface of the sorbent27. Owing to the presence of two hydroxyl group on the HMD ligand structure, it is expected that the extent of its complexation is sensitive to pH28. Thus, the influence of pH on the recovery of 10 μg Cu2+ from 100 ml solutions was studied in the pH range 2.0-8.0. Higher pH values (>8) were not tested due to the possibility of the hydrolysis of octadecyl silica in the disks. As shown in Fig. 2 the Cu2+ ion can be retained quantitatively in the pH range of 5.0-8.0. For subsequent experiments, pH = 7 was chosen as a working pH.
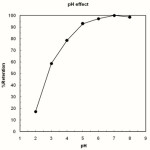 |
Figure 2: Effect of aqueous phase pH on extraction of copper. Copper = 10µg, aqueous phase volume = 100 mL Click here to View figure |
Effect Of Amount of Ligand and Flow Rate of Aqueous Solution
The optimum amount of the ligand for the membrane disks was studied. The results showed that the membrane disks modified with 4-10 mg of HMD are capable of retaining 10μg of Cu2+ ions quantitatively (Fig. 3.). Thus we modified the disks with 5 mg of the ligand for carrying out the subsequent SPE experiments. It is noteworthy that there was no detectable amount of copper retained by the unmodified disk. The effect of flow rate of the sample solution on the retention of copper ions was investigated. As a consequence, in the range of 2-75 ml.min-1 the retention of copper by the membrane disk is not affected by the sample solution flow rate. Thus, the flow rate of the sample solution was maintained at 30 ml. min−1 throughout the experiment. At higher flow rates, the sorption of copper was lower than 90% owing to short residence time of the sample, which resulted in an incomplete retention of copper.
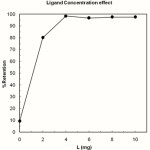 |
Figure 3: Effect of amount of ligand on extraction of copper (II). Copper (II) = 10µg, aqueous phase = 100 mL, pH = 7 (with 0.01 M of phosphate buffer) Click here to View figure |
Choice of Eluent
A satisfactory eluent should effectively elute the retained analytes with small volume, which needed for a high enrichment factor and should not affect the accurate determination of the analytes. In order to choose a proper eluent for the retained Cu2+ ions after the extraction of 10 μg of copper ions in a 100 ml solution by the modified disks, the copper ions were stripped with 5 ml of different concentrations of HNO3, HCl and H2SO4. As given in Table I, it is immediately obvious that among the three different eluent used, 5 ml of 0.07 M HNO3 could accomplish the quantitative elution of copper through the membrane disk, while the use of other eluents were ineffective for the complete elution of copper. The elution studies with different flow rates of 0.07 M HNO3 were also carried out. It was obvious that the recovery did not change significantly up to 30 ml. min-1 which finally was selected for further experiments.
Table 1: Influence of eluents on recovery of copper
| Stripping acid | Recovery (%) | |||||
| Concentration (M) | 0.05 | 0.07 | 0.09 | 0.1 | 0.3 | 0.5 |
| HNO3 | 90.8 | 100.3 | 98.1 | 91.1 | 90.1 | 92.7 |
| HCl | 1 | 1.8 | 5.2 | 14.6 | 26.9 | 41.2 |
| H2SO4 | 1.1 | 3.8 | 25.4 | 45 | 51.3 | 66 |
Aqueous phase: 10 µg Cu2+ in 100 ml, pH = 7 (with 0.01 M of phosphate buffer), amount of ligand = 5 mg, eluent = 5 ml, 0.07 M HNO3
The results of the flow rates reflect the fast complexation – decomplexation reactions of Cu(II) ions with the studied Schiff base.
Analytical performance
Sorption Capacity
The capacity of the modified disks (5 mg HMD) was determined by passing 50 ml of sample solution containing 700 μg of copper and 0.1 M phosphate at pH 7, followed by the determination of the retained copper ions in the eluting solution using FAAS. The maximal capacity of the disk obtained from four replicate measurements as 552.53 (± 18.56) μg of copper on the disk.
Breakthrough Volume
Since breakthrough volume represents the sample volume that can be pre-concentrated without the loss of analyte during elution of the sample, the measurement of breakthrough volume is important in solid phase extraction. The breakthrough volume of the sample solution was tested by dissolving 10 μg of Cu2+ in 25, 50, 100, 250, 500, 1000 and 2000 ml of buffered solution and the recommended procedure was followed. In all cases, the extraction by modified disks was found to be quantitative. Thus, the breakthrough volume for the method should be around 2000 ml. Consequently, by considering the final elution volume of 5 ml and the sample solution volume of 2000 ml, an enrichment factor of around 400 was easily available.
Limit of Detection
Under the selected conditions, eight portions of standard solutions were enriched and analyzed simultaneously following the general procedure. The detection limit based on 3σ of blank is 5.4 ng.L-1.
Effect Of Diverse Ions On Sorption Of Copper
In order to investigate the selective separation and determination of Cu+2 ion from its binary mixtures with diverse metal ions, an aliquot of aqueous solution (100 ml) containing 10 μg Cu+2 and milligram amounts of other cations was taken and the recommended procedure was followed, the results are summarized in Table II. The results show that the copper ions in the binary mixtures are retained almost completely by the modified membrane disk.
Table 2: Separation of copper from binary mixtures a
|
Diverse ions |
Amount taken (each ion, mg) |
Copper recovery (%) |
%RSD (N=4) |
|
Na+ |
100 |
96.3 |
0.5 |
|
K+ |
100 |
95.0 |
0.6 |
|
Ca2+ |
20 |
precipitated |
– |
|
Mg2+ |
20 |
96.5 |
0.3 |
|
Ni2+ |
1 |
94.8 |
1.3 |
|
Co2+ |
1 |
96.0 |
0.6 |
|
Zn2+ |
1 |
92.2 |
0.5 |
|
Mn2+ |
1 |
93.3 |
1.5 |
|
Pb2+ |
1.5 |
precipitated |
– |
|
Na+, K+ ،Mg2+ |
20 |
98.3 |
0.05 |
|
Ni2+, Co2+, Zn2+ |
0.5 |
9.55 |
0.08 |
|
Na+, Ca2+, Mn+2, Ni+2 |
50,0.5b |
94.4 |
0.03 |
aInitial samples contained 10 µg Cu2+ and different amounts of various ions in 100 mL water.
bTaken amount of Na+, Ca2+ was 50 mg and that of Mn+2, Ni+2 ions was 0.5 mg.
Analysis of Artificial and Natural Water Samples
To test the applicability of the developed procedure, it was applied to the extraction and determination of copper ions from some water samples. Tap water (Tehran, taken after 10 min operation of the tap), fall water (Shahandasht Fall, near Tehran), Polur mineral water and synthetic water samples were analyzed. The results for this study are presented in Table III. The recovery of spiked samples is satisfactory reasonable and was confirmed using addition method, which indicates the capability of the system in determination of ions. A good agreement was obtained between the added standards and measured analyte amounts. The recovery values calculated for the added standards were always higher than 90%, thus confirming the accuracy of the procedure and its independence from the matrix effects.
Table 3: Determination of copper ions in real water samples
|
Water sample |
Cu+2 added (µg) |
Copper recovery (µg/l) |
%RSD (N=4) |
|
Tap water |
0 |
0.38 |
0.04 |
|
10 |
10.30 |
0.02 |
|
|
Fall water |
0 |
0.39 |
0.09 |
|
10 |
9.40 |
0.02 |
|
|
Mineral water |
0 |
0.75 |
0.09 |
|
10 |
10.62 |
0.09 |
Aqueous phase: 100 mL sample solution, pH = 7(with 0.01 M of phosphate buffer), flow rate = 30 mL.min-1, amount of ligand = 5 mg, eluent = 5 mL, 0.07 M HNO3. Flow rate of eluent = 8 mL.min-1
Conclusion
A simple, precise and accurate method was developed for selective separation, preconcentration and determination of copper from various complex matrices. Comparative information from some studies on preconcentration of heavy metals by various techniques for the figure of merits is given in Table IV. The detection limit and preconcentration factor of analyte ion are superior to those of preconcentration technique for analysis16, 18, 29-32. The time taken for the separation and analysis of copper in 100 ml sample is at the most 30 min. it can selectively separate Cu+2 ions from various metal ions even when they are present at much higher concentrations. The method can be successfully applied to the separation and determination of copper in real samples.
Table 4: Comparative data from some recent studies on preconcentration
|
Technique |
Analytes |
System |
Eluent |
PF1 |
LOD (µg/L) |
Ref |
|
MF2 |
Pb, Cr, Cu, Ni, Cd |
Cellulose nitrate |
HNO3 |
60 |
0.02-7.8 |
16 |
|
CPE3 |
Cu |
Triton x-114/Cu (II)/ thiamine |
0.1 M NaOH |
10 |
0.29 |
18 |
|
SPE |
Cu, Cd, Pb, Zn, Ni, Co |
Multiwalled carbon nanotubes/APDC |
1M HNO3 in acetone |
80 |
0.03-0.06 |
29 |
|
SPE |
Cd, Cu |
AmberliteXAD-2/ 2-aminothiophenol |
0.5 M HCl |
14-28 |
0.14-0.54 |
30 |
|
MISPE4 |
Cu |
Chitosan-succinate/Cu/ AAPTS |
0.1 M HNO3 |
196 |
0.83 |
31 |
|
SPE |
Cu |
Schiff base |
0.01 M EDTA |
100 |
0.03 |
32 |
|
SPE |
Cu |
Schiff base |
0.07 M HNO3 |
400 |
0.005 |
This work |
Acknowledgments
The authors gratefully acknowledge the support of this work by Tarbiat Moalem University Research council.
References
- Hashemi, P., Bagheri, S., Fat’hi, M. R., Talanta 68, 72 (2005).
- Ashtari, P., Wang, K., Yang, X., Huang, S., Yamini, Y., Anal. Chim. Acta 550, 18(2005) .
- Cassella, R. J., Magalh˜aes, O. I. B., Couto, M. T., Lima, E. L. S., Neves, M. A. F. S., Coutinho, F. M. B., Talanta 67, 121(2005) .
- Acar, O., Talanta 65, 672(2005) .
- Cabon, J. Y., Spectrochim. Acta B 57, 939 (2002).
- Rumori, P., Cerd`a, V., Anal. Chim. Acta 486, 227 (2003).
- Pinto, J. J., Moreno, C., Garc´ıa-Vergas, M., Talanta 64, 562(2004) .
- Meseguer-Lloret, S., Camp´ınas-Falc´o, P., C´ardenas, S., Gallego, M., Valc´arcel, M., Talanta 64, 1030 (2004).
- Szigeti, Z., Bitter, I., Toth, K., Latkoczy, C., Fliegel, D. J., Gunther, D., Pretsch, E., Anal. Chim. Acta 532, 129(2005) .
- Hurst, M. P., Bruland, K. W., Anal. Chim. Acta 546, 68(2005).
- Bermejo-Barrera, P., Moreda-Pi˜neiro, A., Gonz´alez-Iglesias, R., Bermejo- Barrera, A., Spectrochim. Acta B 57, 1951(2002).
- Soylak, M., Saracoglu, S., Divrikli, U., Elci, L., Talanta 66, 1098 (2005).
- Scaccia, S., Zappa, G., Basili, N., J. Chromatogr.A 915, 167 (2001).
- Kend¨uzler, E., Rehber Turker, A., Anal. Chim. Acta 480, 259(2003).
- Lemos, V. A., Baliza, P. X., Talanta 67, 564 (2005).
- Divrikli, U., Kartal, A. A., Soylak, M., Elci, L., J. Hazard. Mater. 145, 459(2007).
- Ghaedi, M., Niknam, K., Shokrollahi, A., Niknam, E., Rajabi, H. R., Soylak, M., J. Hazard. Mater. 155, 121(2008).
- Tabrizi, A. B., J. Hazard. Mater, B 139, 260 (2007).
- Shamsipur, M., Ghiasvand, A. R.,Yamini, Y., Anal. Chem, 71, 4892 (1999).
- Khorrami, A. R., Hashempur, T., Mahmoudi, A., Karimi, A. R., Microchemical Journal 84, 75 (2006).
- Khorrami, A. R., Naeimi, H., Fakhari, A. R., Talanta 64, 13 (2004).
- Yamini, Y., Alizadeh, N., Shamsipur, M., Anal. Chim.Acta 355, 69 (1997).
- Shamsipur, M., Avanes, A., Rofouei, M. K., Sharghi, H.,Aghapour, Gh., Talanta 54, 863 (2001).
- Calligaris, M., Randaccio, R., Wil;inson, G., Gillard, R. D., McCleverty (Eds), Comertensive Coordination Chemistry, Vol. 2, Oxford, London, 1987, Chap. 20.
- Alwood, D. A., Coord. Chem. Rev. 195, 267 (1997).
- Shamsipur, M., Ghiasvand, A. R., Sharghi, H., Naeimi, H., Analytica Chimica Acta 408, 271(2000).
- Poole, f., Poole, S. K., Seibert, d. S., Champman, C. M., J. Chromatogr. B, 689, 245 (1997).
- Pouretedal, H. R., Ph.D . Thesis, ShirazUniversity, Shiraz, Iran, 1998.
- Tuzen, M., Saygi, K. O., Soylak, M., J. Hazard. Mater. 152, 632(2008).
- Lemos, V. A., Baliza, P. X., Talanta 67, 564 (2005).
- Birlik, E., Ers¨oz, A., Denizli, A., Say, R., Analytica Chimica Acta 565, 145(2006).
-
Mashhadizadeh, M. H., Pesteh, M., Talakesh, M., Sheikhshoaie, I., Mazloum Ardakani, M., Karimi, M. A., Spectrochimica Acta Part B 63, 885(2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









