Chemical Compounds and Antibacterial Activity of Tephrosia Toxicaria Pers.
Angela Martha Campos Arriaga1, Francisca Renata Lopes Da Silva1, Maria Valdeline Sousa Texeira1, Italo Gomes Pereira1, Marcos Reinaldo Da Silva1, Jair Mafezoli1, Gilvandete Maria Pereira Santiago1, Jackson Nunes E Vasconcelos2, Raimundo Braz-Filho3, José Galberto Martins Da Costa4, Edinardo F.F. Matias4 and Fabiola Fernandes Galvão Rodrigues4
1Department of Organic and Inorganic Chemistry, Science Center, Federal University of Ceará, CP 12200, 60021-940, Fortaleza-CE, Brazil.
2Federal Institute of Education, Science and Technology of Ceará, Tiangua Campus, CE Highway 187 sn, Airport, 62320-000, Tiangua-CE, Brazil.
3Laboratório de Ciências Químicas, University Estadual do Norte Fluminense Darcy Ribeiro, 28013-602, Campos dos Goytacazes-RJ, Brazil.
4University of Cariri, Research Laboratory for Natural Products, Rua Cel. Antônio Luís 1161, 63105-000, Crato-CE, Brazil.
Corresponding Author E-mail: ang@ufc.br
DOI : http://dx.doi.org/10.13005/ojc/330504
Eight known compounds were isolated from the shrub Tephrosia toxicaria. Among them, 6,7-dimethoxy-chromone (1), was described by the first time for this genus, villosinol (2), which had been previously reported without its 13C-NMR data and sumatrol (3), which has its 13C-NMR data corrected. The antibacterial activity of Tephrosia toxicaria extract and obovatin (6), deguelin (7), 12a-hydroxy-α-toxicarol (8), 12a-hydroxy-rotenone (10), and tephrosin (11) is also described.
KEYWORDS:Tephrosia Toxicaria; Flavonoids; 13C NMR Data; Antibacterial Activity
Download this article as:| Copy the following to cite this article: Arriaga A. M. C, Silva F. R. L. D, Texeira M. V. S, Pereira I. G, Silva M. R. D, Mafezoli J, Santiago G. M. P, Vasconcelos J. N. E, Braz-Filho R, Costa J. G. M. D, Matias E. F. F, Rodrigues F. F. G. Chemical Compounds and Antibacterial Activity of Tephrosia Toxicaria Pers. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Arriaga A. M. C, Silva F. R. L. D, Texeira M. V. S, Pereira I. G, Silva M. R. D, Mafezoli J, Santiago G. M. P, Vasconcelos J. N. E, Braz-Filho R, Costa J. G. M. D, Matias E. F. F, Rodrigues F. F. G. Chemical Compounds and Antibacterial Activity of Tephrosia Toxicaria Pers. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=38936 |
Introduction
Tephrosia toxicaria Pers. (Fabaceae), also referred as T. sinapou (Buc´hoz), is a shrub popularly known as “timbó de caiena” in Ceará state (Northeast of Brazil) where it is used as pesticide and fishing poison1-2. The phytochemical studies of Tephrosia genus revealed compounds with anticinoceptive, larvicidal and antiinflammatory activities3,4. Previous investigations of T. toxicaria led to the identification of flavonoids, mainly rotenoids1-2,5-7. In the present work, we report the isolation of eight known compounds, including the chromone, 6,7-dimethoxy-chromone, 1 described for the first time for this genus, the flavonoid, villosinol, 2 which has been previously reported without its 13C-NMR data and sumatrol, 3 which has its 13C-NMR data corrected. In addition, as a support of use of alternative source in the treatment of bacterial infections, we describe the antibacterial activity of ethanolic extract from its roots and of some its compounds.
Material and Methods
Plant Material
Pods and roots of T. toxicaria Pers. were collected in Guaraciaba do Norte (Ceará state, Brazil). A voucher specimen (#32139) is kept at the Herbarium Prisco Bezerra, Universidade Federal do Ceará – Brazil.
Methods
The powdered pods, air-dried (180.0 g), were extracted with 95% EtOH at and provided the extract TTVE (26.0 g). An aliquot (8.7g) of TTVE was fractionated by Si gel column (Merck 60-120 60 Mesh) using hexane, CH2Cl2 and EtOAc as solvents. The fraction eluted with CH2Cl2 (69.0 mg) furnished, after further Si gel column, 6.7 mg of 6a,12a-desydro-α-toxicarol8, 4. The fraction eluted with EtOAc (800.0 mg) was purified by Si gel column, furnished 19.9 mg of luteolin9, 5. The powdered roots (500.0 g) were extracted with ethanol to afford TTRE (14.2 g). TTRE was chromatographed on a Si gel column with gradient mixture of hexane/CH2Cl2. Fractions eluted with hexane/CH2Cl2 (1:1) yielded 13.2 mg of obovatin10, 6, and the fractions eluted with CH2Cl2 furnished 9.9 mg of deguelin8, 7. Another portion of roots (649.0 g) was extracted in a Soxhlet system with water. After liophilization, the material (5.4 g) was extracted with ethyl acetate and yielded 2.1 g of organic fraction. This fraction was chromatographed on Sephadex LH-20 using EtOAc:MeOH (1:1). The fractions obtained were submitted to HPLC RP-18 (MeOH/formic acid 0.1% 4:1) and provided pure compounds villosinol11 (2, 5.0 mg), 12a-hydroxy-α-toxicarol8 (8, 32.8 mg), sumatrol12 (3, 3.6 mg), α–toxicarol8 (9, 13.0 mg), 12a-hydroxyrotenone13 (10, 27.8 mg), tephrosin14 (11, 62.0 mg), and 6,7-dimethoxy-chromone15 (1, 2.0 mg).
All compounds were identified by 1H-NMR, 13C-NMR and 2D NMR analysis and comparison with those reported in the literature.
Antibacterial Activity
Microorganisms
The following strains used in this study were provided by the Oswaldo Cruz Foundation – FIOCRUZ: K. pneumoniae ATCC 10031; P. aeruginosa ATCC 15442; S. mutans ATCC 0046; S. aureus ATCC 6538. Strains isolated from clinical material of Escherichia coli Ec 27 and Staphylococcus aureus Sa 358 were also used. The bacteria were activated in Brain Heart Infusion (BHI, Himedia laboratories PVT. LTD., Mumbai, INDIA) for 24 h at 35°C.
Antibacterial Test (MIC) and Resistance Modulation Bacterial
MIC (minimal inhibitory concentration) was determined in a microdilution assay16 utilizing an inoculum of 100 µL of each strain, suspended in BHI broth up to a final concentration of 105 CFU/mL in 96-well microtiter plates, using serial dilutions (1:1). Each well received 100 µL of each (roots ethanolic extract (TTRE) and of the compounds 6, 7, 8, 10 and 11). The concentrations of the extract and organic compounds varied 512 – 8 µg/mL. MICs were recorded as the lowest concentrations required to inhibit growth.
The minimum inhibitory concentration for antibiotics was determined in BHI by the microdilution test, using suspensions of 105 CFU/mL and a drug concentration ranging from 2,500 to 2,4 μg/mL (1:1 serial dilutions). MIC was defined as a lower concentration at which growth was observed. For evaluation of the absence and presence of deguelin (7) and 12a-hydroxy-α-toxicarol (8) in P. aeruginosa and Staphylococcus aureus modulators of antibiotic resistance, a MIC of the antibiotics was determined in the presence or absence of subunits and as plates were incubated for 24 h at 37ºC. Each antibacterial test for MIC determination was performed in triplicate.
Results and Discussions
It is worth to mention that villosinol, 2 has its 13C-NMR data being reported for the first time, and that the 13C-NMR assignment for sumatrol, 3 was corrected through bidimensional NMR analysis.
Villosinol (2). C23H22O8, 1H-NMR (500 MHz, CDCl3) δH: 1.74 (s, 3H, CH3-8’), 2.84 (dd, J = 15.0 and 7.5 Hz, 1H, H-4’a), 3.20 (dd, J = 15.0 and 9.5 Hz, 1H, H-4’b), 3.76 (s, 3H, 2-OCH3), 3.83 (s, 3H, 3-OCH3), 4.18 (s, 1H, 12a-OH), 4.47 (d, J = 12.0 Hz, 1H, Heq-6), 4.55 (sl, 1H, H-6a), 4.58 (dd, J = 12.0 and 2.5 Hz, 1H, Hax-6), 4.93 (s, 1H, H-7’), 5.05 (s, 1H, H-7’), 5.20 (t, J = 8.5 Hz, 1H, H-5’), 6.04 (s, 1H, H-10), 6.49 (s, 1H, H-4), 6.71 (s, 1H, H-1), 11.83 (s, 1H, 11-OH). 13C-NMR (125 MHz, CDCl3) δC: 194.4 (C-12), 170.4 (C-9), 166.1 (C-11), 156.1 (C-7a), 151.6 (C-3), 148.7 (C-4a), 144.4 (C-2), 143.1 (C-6’), 113.2 (C-7’), 109.7 (C-1), 109.0 (C-1a), 105.0 (C-8), 101.4 (C-4), 100.2 (C-11a), 92.5 (C-10), 88.7 (C-5’), 75.8 (C-6a), 67.1 (C-12a), 63.9 (C-6), 56.7 (2-OCH3), 56.2 (3-OCH3), 30.8 (C-4’), 17.3 (C-8’).
Sumatrol (3). C23H22O7. mp 213.6–215.1 ºC. 1H-NMR (500 MHz, CDCl3) δH: 1.75 (s, 3H, CH3-8’), 2.86 (dd, J = 15.0 and 8.0 Hz, 1H, H-4’a), 3.24 (dd, J = 15.0 and 9.5 Hz, 1H, H-4’b), 3.79 (s, 3H, 2-OCH3), 3.82 (s, 3H, 3-OCH3), 3.85 (d, J = 4.0 Hz, 1H, H-12a), 4.17 (d, J = 12.0 Hz, 1H, Heq-6), 4.59 (dd, J = 12.0 and 3.0 Hz, 1H, Hax-6), 4.88 (t, J = 3.0 Hz, 1H, H-6a), 4.93 (s, 1H, H-7’), 5.06 (s, 1H, H-7’), 5.20 (t, 1H, J = 8.5 Hz, 1H, H-5’), 6.02 (s, 1H, H-10), 6.46 (s, 1H, H-4), 6.87 (s, 1H, H-1), 12.42 (s, 1H, 11-OH). 13C-NMR (125 MHz, CDCl3) δC: 193.8 (s, C-12), 169.6 (s, C-9), 166.4 (s, C-11), 156.5 (s, C-7a), 150.1 (s, C-3), 147.6 (s, C-4a), 144.4 (s, C-2), 143.2 (s, C-6’), 112.8 (t, C-7’),110.9 (d, C-1), 105.0 (s, C-1a), 104.3 (s, C-8), 101.5 (s, C-11a), 101.4 (d, C-4), 92.1 (d, C-10), 88.4 (d, C-5’), 72.0 (d, C-6a), 66.3 (t, C-6), 56.7 (q, 2-OCH3), 56.2 (q, 3-OCH3), 44,0 (d, C-12a), 30.9 (t, C-4’), 17.3 (q, C-8’).
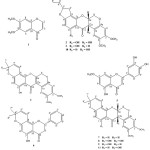 |
Figure 1: Compounds isolated from T. toxicariaClick here to View figure |
In vitro Antibacterial Activity
The MICs of Tephrosia toxicaria extract, TTRE, obovatin, (6), deguelin (7), 12a-hydroxy-α-toxicarol (8), 12a-hydroxy-rotenone (10), and tephrosin (11) against six bacterial strains are presented in Table 1. All tested compounds and extract showed antimicrobial activity against Gram-positive and Gram-negative bacteria, with the best effect of 12a-hydroxy-α-toxicarol against to the grown of Gram-positive S. aureus 358 with MIC 256 µg/mL, while Deguelin is responsible for the best result, the Gram-negative bacteria, P. aeruginosa was inhibited at 64 µg/mL.
Table 2 shows the results concerning the modulation tests of bacterial resistance to aminoglycosides. When 7 and 8 compounds are combined with the antibiotic amikacin, tested against P. aeruginosa and S. aureus strains, the isolated and combined MIC values were the same. Synergism was observed in the combinations of 7 with gentamicin against the two bacterial strains used in the test and in the combination of 7 with neomycin against P. aeruginosa, characterized by reduction of MIC by 50% compared to MIC of the antibiotics tested alone. Only the 8 combination with neomycin against S. aureus showed antagonism, increasing the MIC by 50% compared to the MIC of the neomycin tested alone.
Some natural products of origin plant and phytochemicals are known to have antimicrobial properties, which may be of great importance in treatment against infections. With the increased incidence of antibiotic resistance, alternative natural plant products may be of interest. 17. Several studies have been conducted in different countries, demonstrating the efficacy of this type of treatment. Many natural products of plants were evaluated not only for direct antimicrobial activity, but also as resistance modifying agents18,19.
Various chemical compounds (synthetic or natural) have direct antibacterial activity against many species, expanding the activity of an antibiotic, reversing the natural resistance of bacteria to specific antibiotics, causing the inhibiting the active efflux of antibiotics through the plasma membrane and / or elimination of plasmids. Potentiation of antibiotic activity or reversion of resistance to antibiotics allows the classification of these compounds as modifiers of antibiotic activity20,21.
The mechanism described as “synergistic multi-effect targeting” or “Herbal shotgun” is a possible strategy that explains modulatory effects and refers to the use of plants and drugs in an approach using various-substance combinations, which may not only affect a single target, but several ones, where the different therapeutic components contribute in a synergistic-agonistic effect. This approach is not only for combinations of extracts: Combinations between complex mixtures (extracts and/or oil) and compounds chemical isolated synthetic or naturals or antibiotics are also possible 22,23.
Table 1: Values of the minimal inhibitory concentration (MIC) (μg/mL) of Tephrosia toxicaria extract and obovatin (6), deguelin (7), 12a-hydroxy-α-toxicarol (8), 12a-hydroxy-rotenone (10), and tephrosin (11)
|
E. coli (27) |
K. pneumoniae (10031) |
P. aeruginosa (15442) |
S. mutans (0046) |
S. aureus (6538) |
S. aureus (358) |
|
|
TTRE |
512 |
512 |
≥1024 |
512 |
512 |
≥1024 |
|
6 |
≥1024 |
≥1024 |
≥1024 |
512 |
512 |
≥1024 |
|
7 |
≥1024 |
512 |
64 |
≥1024 |
512 |
512 |
|
8 |
512 |
512 |
≥1024 |
≥1024 |
512 |
256 |
|
10 |
512 |
512 |
512 |
512 |
512 |
≥1024 |
|
11 |
512 |
512 |
512 |
≥1024 |
512 |
≥1024 |
Table 2: MIC values (μg/mL) of aminoglycosides in the absence and presence of compounds 7 and 8 in P. aeruginosa and Staphylococcus aureus.
|
Antibiotics |
P. aeruginosa (15442) |
S. aureus (358) |
||
|
MIC alone |
MIC combined (7) |
MIC alone |
MIC combined (8) |
|
|
Gentamicin |
64 |
32 |
64 |
32 |
|
Amicacin |
32 |
32 |
64 |
64 |
|
Neomycin |
32 |
16 |
32 |
64 |
Conclusion
In conclusion, the prospective health-promoting effects of plant secondary metabolites have encouraged the research about their chemical constitution and their potential to treat several diseases, among them bacterial infection. The plant substances and their extracts display rarely toxic side effects when compared in treatments with conventional drugs, so these results could be leads for the development of new antibacterial agent, or the compounds from T. toxicaria even may be used associates with standard antibiotics.
Authors’ Contribuitions
FRLS, MVST, JNV, JQL (Master and PhD sudents), IGP (Graduate student) contributed running the laboratory work and drafted the paper; AMCA, JM, MRS, RBF, GMPS did the analysis and interpretation of data of RMN, critical revision of the manuscript. JGMC, EFFM and FFGR contributed with the microbiological tests. All the authors have read the final manuscript and appoved the submission.
Acknowledgement
The authors are grateful to CNPq, CNPq/Pronex and CAPES for financial support and fellowships.
References
- Vasconcelos, J. N.; Lima, J. Q.; Lemos, T. L. G.; Oliveira, M. C. F.; Almeida, M. M. B.; Andrade-Neto, M.; Mafezoli, J.; Arriaga, A. M. C.; Santiago, G. M. P.; Braz-Filho, R. Quim. Nova, 2009, 32, 382-386
CrossRef - Vasconcelos, J. N.; Santiago, G. M. P.; Lima, J. Q.; Mafezoli, J.; Lemos, T. L. G.; Silva, F. R. L.; Lima, M. A. S.; Pimenta, A. T. A.; Braz-Filho, R.; Arriaga, A. M. C.; Cesarin-Sobrinho, D. Quim Nova, 2012, 35, 1097-1100
CrossRef - Chen, Y.; Yan, T.; Gao, C.; Cao, W.; Huang, R. Molecules, 2014, 19, 1432-1458
CrossRef - do Val, D. R.; Bezerra, M. M.; Silva, A. A. R.; Pereira, K. M. A.; Rios, L. C.; Lemos, J. C.; Arriaga, N. C.; Vasconcelos, J. N.; Benevides, N. M. B.; Pinto, V. P. T.; Cristino-Filho, G.; Brito, G. A. C.; Silva, F. R. L.; Santiago, G. M. P.; Arriaga, A. M. C.; Chaves, H. V. European Journal of Pain, 2014, 18, 1280-1289
CrossRef - Pancharoen, O.; Petveroj, S.; Phongpaichit, S. Songklanakarin J. Sci Technol. 2007, 29, 151-156
- Arriaga, A. M. C.; Malcher, G. T.; Lima, J. Q.; Magalhães, F. E. A.; Gomes, T. M. B. M.; Oliveira, M. C. F.; Andrade-Neto, M.; Mafezoli, J.; Santiago, G. M. P. J. Essent. Oil Res. 2008, 20, 450-451
CrossRef - Arriaga, A. M. C.; Lima, J. Q.; Vasconcelos, J. N.; Oliveira, M. C. F.; Andrade-Neto, M.; Santiago, G. M. P.; Uchoa, D. E. A.; Malcher, G. T.; Mafezoli, J.; Braz-Filho, R. Magnetic Resonance in Chemistry, 2008, 47, 537-540
CrossRef - Andrei, C. C.; Vieira, P. C.; Fernandes, J. B.; da Silva, M. F. G. F.; Rodrigues Filho, E. Phytomchemistry, 1997, 46, 1081-1085
CrossRef - Agrawal, P. K. Carbon-13 NMR of flavonoids; Elsevier, 1989
- Andrei, C. C.; Ferreira, D. T.; Faccione, M.; de Moraes, L. A. B.; de Carvalho, M. G.; Braz-Filho, R. Phytochemistry, 2000, 55, 799-804
CrossRef - Krupadanam, G. L. D.; Sarma, P. N.; Srimannarayana, G.; Subbaroa, N. V. Tetrahedron Lett., 1977, 24, 2125-2128
CrossRef - Jang, D. S.; Park, E. J.; Kang, Y.; Hawthorne, M. E.; Vigo, J. S.; Graham, J. G.; Cabieses, F.; Fong, H. H. S.; Mehta, R. G.; Pezzuto, J. M.; Kinghorn, A. D.; J. Nat. Prod., 2003, 66, 1166-1170
CrossRef - Phrutivorapongkul, A.; Lipipun, V.; Ruangrunngsi, N.; Watanabe, T.; Ishikawa, T. Chem. Pharm. Bull., 2002, 50, 534-537
CrossRef - Ahmad, V. U.; Ali, Z.; Hussauni, S. R.; Iqbal, F.; Zahid, M.; Abbas, M.; Saba, N. Phytochemical Commun., 1999, 70, 443-445
- Romussi, G.; Ciarallo, G. J. Heterocyclic Chem., 1976, 13, 211-220
CrossRef - Javadpour, M. M.; Juban, M. M.; Jo, W. C.; Bishop, S. M.; Alberty, J. B.; Cowell, S. M.; Becker, C. L.; McLaughlin, M. L. J. Med. Chem., 1996, 39, 3107-3113
CrossRef - Matias, E. F. F.; Santos, K. K. A.; Almeida, T. S.; Costa, J. G. M.; Coutinho, H. D. M. Rev. Bras. Biocienc. 2010, 8, 294-298
- Matias, E. F. F.; Alves, E. F.; Santos, B. S.; Souza, C. E. S.; Ferreira, J. V. A.; Lavor, A. K. L. S.; Figueredo, F. G.; Lima, L. F.; Santos, F. A. V.; Peixoto, F. S. N.; Colares, A. V.; Boligon, A. A.; Saraiva, R. A.; Athayde, M. L.; Rocha, J. B. T.; Menezes, I. R. A.; Coutinho, H. D. M.; Costa, J. G. M. J. Evid. Based. Complementary Altern. Med., 2013a, 1-7
- Matias, E. F. F.; Santos, K. K. A.; Falcão-Silva, V. S.; Siqueira-Júnior, J. P.; Costa, J. G. M.; Coutinho, H. D. M. Indian J. Med. Res., 2013b, 137, 178-182
- Coutinho, H. D. M.; Costa, J. G. M.; Lima, E. O.; Falção-Silva, V. S.; Siqueira-Júnior, J. P. BMC Complement. Altern. Med. 2009a, 9, 13
CrossRef - Coutinho, H. D. M.; Costa, J. G. M.; Lima, E. O.; Siqueira-Júnior, J. P. Comp. Immunol. Microbiol. Infect. Dis., 2010, 33, 467-471
CrossRef - Hemaiswarya, S. H.; Kruthiventi, A. K.; Doble, M. Phytomedicine, 2008, 15, 639-652
CrossRef - Wagner, H.; Ulrich-Merzenich, G. Phytomedicine, 2009, 16, 97-110
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









