Anti-Melanogenic Activities of Sargassum Muticum Via MITF Downregulation
Min-Jin Kim1, Kwang Hee Hyun2, Ji-Hye Hyun2, Sobin Im2, Jihan Sim2, Nam Ho Lee1 and Chang-Gu Hyun1
1Cosmetic Sciences Center, Department of Chemistry and Cosmetics, Jeju National University, Jeju63243, Korea.
2Helios Co., Ltd., Sanchundan Dong-gil 16, Jeju 63243, Korea.
Corresponding Author E-mail: cghyun@jejunu.ac.kr
DOI : http://dx.doi.org/10.13005/ojc/330401
Sargassummuticum is a seaweed used in traditional foods on Jeju Island in Korea. The present study tested the effects of S. muticum on melanin biosynthesis in B16F10 melanoma cells. We obtained S.muticum extract by treating the weed with 70% ethanol and then extracted it with ethyl acetate (EtOAc). The results indicated that the S. muticumEtOAc fraction (SME) downregulated melanin content and cellular tyrosinase activity in a concentration-dependent manner. To clarify the target of SME activity in melanogenesis, we performed Western blotting for the key melanogenic enzymes, tyrosinase, dopachrometautomerase (DCT), tyrosinase-related protein (TRP)-1and microphthalmia-associated factor (MITF). SME inhibited the expression of these enzymes in a concentration-dependent manner. In summary, SME degrades MITF, thereby suppressing melanogenic enzyme activity and melanin production. These findings suggest that SME may be useful a skin-lightening agent to preventhyperpigmentation-related diseases.
KEYWORDS:Melanin; melanogenesis; microphthalmia-associated factor; Sargassummuticum; tyrosinase
Download this article as:| Copy the following to cite this article: Kim M. J, Hyun K. H, Hyun J. H, Im S, Sim J, Lee N. H, Hyun C. G.Anti-Melanogenic Activities of Sargassum Muticum Via MITF Downregulation. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Kim M. J, Hyun K. H, Hyun J. H, Im S, Sim J, Lee N. H, Hyun C. G.Anti-Melanogenic Activities of Sargassum Muticum Via MITF Downregulation. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=35130 |
Introduction
Human skin is damaged by repeated exposure to the harmful effects of solar ultraviolet (UV) radiation and environmental pollutants and, therefore, requires a large number of endogenous systems to protect against damage due to these1,2. Melanin, a dark pigment in the skin, is one of these endogenous systems, and it functions as a broadband UV absorbent, antioxidant, and radical scavenger. Thus, melanin is the most important photoprotectiveand reactive oxygen species-protective factor for human skin health3,4. However, excessive melanin production results in skin disorders, such as melanoderma, melasma, age spots, and freckles5,6. Therefore, strategies to modulate melanin production are strongly desired in the medicinal andcosmetic fields for skin beautification and abnormal skin pigmentation treatment, respectively7-9.
Melanin is synthesized in the melanosomes of melanocytes by a family of melanocyte-specific enzymes, includingtyrosinase, dopachrometautomerase (DCT), tyrosinase-related protein (TRP)-1. These three enzymes are regulated by microphthalmia-associated transcription factor (MITF), a critical transcription factor in melanogenic processes10-13. Therefore, some inhibitors of melanogenesis-associated enzymes, such as arbutin and resveratrol, have been used in cosmetic applications14,15.
Sargassummuticum, the raw material for “mom-kuk” (a traditional food), has been commonly consumed as a popular marine vegetable for more than 500 years on Jeju Island in Korea. This brown alga is widely distributed along the shores of the island, and it is one of the top economically important seaweeds in Korea. We and other researchers have reported that S. muticum has hair growth, anti-inflammatory, and anti-wrinkle properties16-18. Nevertheless, its anti-melanogenic activity remains uncharacterized. The aim of this study was to determine the anti-melanogenic effects of the ethyl acetate (EtOAc) fraction of S. muticum extract (SME) by measuring melanin synthesis in SME-treated B16F10 melanoma cells.
Materials and Methods
Sme Preparations
S.muticum was collected in December 2015 from the waters surrounding Jeju Island, Korea. For extraction, the seaweed was washed with water and dried at room temperature for 10 days. The dried seaweed was ground into a fine powder using a blender. The dried powder (1 kg) was extracted with 70% ethanol (EtOH, 4 L) for 1 day and then evaporated under vacuum. The evaporated EtOHextract (10 g) was suspended in fresh water (1 L) and fractionated with ethyl acetate (EtOAc, 1 L). The yield and recovery of SME were 10.67g and 10.67%, respectively.
Cell Culture and Proliferation
B16F10 murine melanoma cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM) that was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin serum (GIBCO BRL Life Technologies, Grand Island, NY, USA). The cells were routinely maintained in air containing 5% CO2 at 37°C. A viability assay for the B16F10 murine melanoma cells was assessedusing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). In brief, themelanomacells (1 × 104 cells/100 µL/well) were seededinto 96-wellmicroplatesand treated with various concentrations (3.125 to 25 µg/mL) of SME for 24 h at 37°C in a 5% CO2 atmosphere. The resulting violet formazan precipitate was solubilized by adding ethanol-dimethyl sulfoxide (1:1 mixture solution). The absorbance was measured at 570 nm using a BioTekPowerWave HT microplate spectrophotometer (Winooski, VT, USA).
Measurement of Relative Melanin Content and Intracellular Tyrosinase Activity
B16F10 melanoma cells were seeded at a density of 2 × 104cells/well on 6-well culture plates and incubated at 37°C under a 5% CO2 atmosphere for 18 h. The B16F10 cells were then treated with or without α-melanocyte-stimulating hormone (α-MSH), melasolv (40 μM), and SME (1.25, 2.5, and 5 μg/mL) at 37°C for 72 h. To determine melanin content, the culture cells were lysed in 67 mM sodium phosphate buffer (pH 6.8) containing 0.2 mMphenylmethylsulfonylfluoride (PMSF)and 1% Triton-X 100 and centrifuged at 12,000 rpm for 30 min at 4°C. The cell pellets were then lysed with100 μL of 1Nsodium hydroxide for 15 min at 95°C. The absorbance of the cell lysateswas measured at 405 nm. For monitoring intracellular tyrosinase activity,B16F10 cells (2.0 × 104 cells/well) were pre-incubated in a 60 mm dish and then incubated at 37°C in a humidified atmosphere with 5% CO2 for 18 h. The cultured cells were lysed with 50 mMPBS buffer (pH 7.5) containing 1.0% Triton X-100 and 0.1 mMPMSF, and the lysates were collected via centrifugation for 15 min at 10,000 rpm. The cell extracts were mixed with 15 mMl-3,4-dihydroxyphenylalanine (L-DOPA) and incubated for 1 h at 37°C. Absorbance was measured at 475 nm using a BioTekPowerWave HT microplate spectrophotometer.
Western Blot Analysis
Cell extract containing 30 mg solubilized protein was separated in a 4–12% Bis-Tris mini gel (Invitrogen, Carlsbad, CA, USA) and transferred to a nitrocellulose membrane. After incubation with the blocking buffer [Tris-buffered saline containing Tween 20(TTBS)with 5% skim milk] for 24 h, the membranes were incubated overnight at 4°C with TTBS-diluted (1:1000) primary antibodies (TRP-1, TRP-2, tyrosinase, and MITF; Santa Cruz Biotechnology). Then, the membranes were incubated with secondary peroxidase-conjugated goat immunoglobulin G antibody (1:5000). The blotted antibodies were visualized using an enhanced chemiluminescence solution and exposed to an X-ray film. The film was scanned to quantify the intensity of bands.
Statistical Analysis
All data are expressed as means ± standard deviation. Significant differences between the groups were determined using the Student’s t-test. P-values less than 0.05 were considered significant.
Results and Discussion
Chemical agents such as hydroquinone, arbutin, kojic acid, and phenylthiourea currently used to treat pigment disorders have several drawbacks19. The use of hydroquinone, an aglycone of arbutin, is controversial due to its toxicity and mutagenic properties and has been banned in several countries. The use of kojic acid has also been limited in cosmetics owing to its toxicity and instability20,21. Therefore, natural ingredientsthat inhibit melanin production have generated interest for use as components in cosmetic agents.In our ongoing screening program designed to identify the anti-melanogenic potential of seaweeds22,23, we investigated the effects of S.muticum on melanin biosynthesis in B16F10 melanoma cells.
The in vitro safety of natural extracts is the first considerationin the formulation of cosmetic ingredients.Therefore, we initially measured the cell viability of SME in B16F10 murine melanoma cells using an MTT assay. As shown in Figure 1, B16F10 murine melanoma cells were treated with various concentrations of SME (3.125–12.5μg/mL). Cell viability was more than 85%after treatment with 6.25 μg/mL SME. Therefore, we used SME at doses of 1.25–5.0μg/mL to determine the melanin content and intracellulartyrosinaseactivity in B16F10 murine melanoma cells treated with SME.
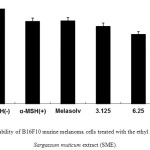 |
Figure 1: Cell viability of B16F10 murine melanoma cells treated with the ethyl acetate fraction of Sargassum muticum extract (SME). |
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed after the incubation of B16F10 murine melanoma cells treated with various concentrations of SME (3.125, 6.25, and 12.5 μg/mL) for 24 h at 37°C in a 5% CO2incubator. The absorbance was measured at 570 nm using a BioTekPowerWave HT microplate spectrophotometer (Winooski, VT, USA).
Themelanin content of the B16F10 murine melanoma cells was measured after SME treatment, and melasolv was used as apositive control because it inhibitsmelaninsynthesis22. B16F10 murine melanoma cells were exposed to various concentrations of SME for 72 h. Ourresults showed that compared with control cells, SME-treatedcells showed decreased pigmentation in a concentration-dependent manner and themelasolv-treated cells were also markedly less pigmented(Figure 2A). In addition, SME treatment significantly reduced cellular tyrosinase activity in a concentration-dependent manner (Figure 2B).
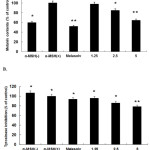 |
Figure 2: Inhibitory effect of SME on melanin content (A) and cellular tyrosinase activity (B) in B16F10 murine melanoma cells. |
B16F10 murine melanoma cells (2.0 × 104 cells/mL) were pre-incubated for 18 h, and melanin content was measured after the incubation of B16F10 cells treated with α-melanocyte-stimulating hormone (α-MSH; 100 nM), melasolv (40 μM), and SME (1.25, 2.5, and 5 μg/mL) for 72 h at 37°C in a 5% CO2incubator. Absorbances were measured at 405 nm and 475 nm using a spectrophotometer.
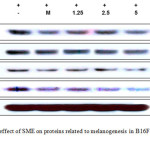 |
Figure 3: Inhibitory effect of SME on proteins related to melanogenesis in B16F10 murine melanoma cells. |
B16F10 murine melanoma cells (1.0 × 105 cells/mL) were pre-incubated for 18 h and then stimulated with α-MSH (100 nM) in the presence of melasolv (40 μM) and SME (1.25 to 5 μg/mL) for 24 h. Protein levels were determined using immunoblotting method. MITF, microphthalmia-associated factor; TRP, tyrosinase-related protein
Melanin production in human skin is a complex process involving many enzymatic cascades, includingthose of tyrosinase, TRP-1, and TRP-2. Therefore, to more precisely examine the possible mechanism of the inhibitoryeffect of SME on melanogenesis10-12, weperformed Western blot analysis of SME-treated B16F10 murine melanoma cells.As shown in Figure3, SME at 1.25–5.0μg/mL reduced the expression levels of TRP-1,TRP-2, and tyrosinasein a concentration-dependent manner at 48 h. Furthermore, because these melanogenicenzymes aretranscriptionally regulated by MITF, which leads tomelanogenesis, we next performed Westernblotting to determine the expression levels of MITF. The results showed thatSME dramatically and concentration-dependently inhibited MITFexpression in B16F10 murine melanoma cells (see Figure3). These results clearly indicated that the anti-melanogenesiseffect of SME is accompaniedby corresponding downregulation of MITF expression, whichconsequently reduces melanogenic protein expression.
Conclusion
In conclusion, the present study is the first to demonstratethat SME treatment inhibits melanogenesis by inhibiting MITF, which leads to the downregulationof melanogenicenzymes, such as tyrosinase, TRP1, and TRP2, and reduces thesynthesisof tyrosinase and melanin. These resultsindicate that SME is safe for use as a skin-whitening agentto enhance skin esthetics and treat hyperpigmentationdisorders.
Acknowledgements
This work was supported by the Academic and Research Institutions R&D Program (C0268105) funded by the Small and Medium Business Administration (SMBA, Korea).
References
- Marionnet, C.; Tricaud, C.; Bernerd, F.; Int. J. Mol. Sci. 2014.16, 68-90.
- Poljšak, B,; Fink, R.;Oxid.Med. Cell Longev.2014, 2014, 671539.
- Roberts, N.B.; Curtis, S.A.; Milan, A.M.;Ranganath, L.R. JIMD Rep. 2015, 24, 51-66.
CrossRef - D’Mello, S.A.; Finlay, G.J.;Baguley, B.C.;Askarian-Amiri, M.E.;Int. J. Mol. Sci. 2016,17, E1144.
CrossRef - Huang, H.C.; Ho, Y.C.; Lim, J.M.; Chang, T.Y.; Ho, C.L.; Chang, T.M.;Int. J. Mol. Sci. 2015, 16, 10470-10490
CrossRef - Huang, H.C.; Wei, C.M.;Siao, J.H.; Tsai, T.C.;Ko, W.P.; Chang, K.J.;Hii, C.H.; Chang, T.M.; Evid.Based Complement.Alternat.Med. 2016, 2016, 5860296.
- Yoon, H.S.;Ko,H.C.; Kim, S.S.; Park, K.J.; An, H.J.; Choi, Y.H.; Kim, S.J.; Lee, N.H.; Hyun, C.G.; Nat. Prod.Commun.2015, 10, 389-392.
- Yoon, W.J.; Ham, Y.M.; Yoon, H.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G.;Nat. Prod.Commun.2013, 8, 1359-1362.
- Yoon, W.J.; Kim, M.J.; Moon, J.Y.; Kang, H.J.; Kim, G.O.; Lee, N.H.; Hyun, C.G.;J. Oleo Sci. 2010, 59, 315-319.
CrossRef - Tuerxuntayi, A.; Liu, Y.Q.;Tulake, A.;Kabas, M.;Eblimit, A.;Aisa, H.A.;BMC Complement.Altern. Med. 2014,14, 166.
CrossRef - Kim, Y.M.; Cho, S.E.;Seo, Y.K.;Life Sci. 2016, 162, 25-32.
CrossRef - Baek, S.H.; Lee, S.H.;Exp.Dermatol. 2015, 24, 761-766.
CrossRef - Qiao, Z.; Koizumi, Y.; Zhang, M.;Natsui, M.; Flores, M.J.;Gao, L.;Yusa, K.;Koyota, S.; Sugiyama, T.;Biosci.Biotechnol.Biochem.2012, 76, 1877-1883.
CrossRef - Okura, M.; Yamashita, T.; Ishii-Osai, Y.; Yoshikawa, M.;Sumikawa, Y.; Wakamatsu, K.; Ito, S.;J.Dermatol. Sci. 2015,80, 142-149.
CrossRef - Lee, T.H.;Seo, J.O.; Do, M.H.;Ji, E.;Baek, S.H.; Kim, S.Y.;Biomol.Ther. (Seoul) 2014,22, 431-437.
CrossRef - Song, J.H.;Piao, M.J.; Han, X.; Kang, K.A.; Kang, H.K.; Yoon, W.J.;Ko, M.H.; Lee, N.H.; Lee, M.Y.;Chae, S.; Hyun, J.W.;Mol. Med. Rep. 2016, 14, 2937-2944.
CrossRef - Kang, J.I.;Yoo, E.S.; Hyun, J.W.;Koh, Y.S.; Lee, N.H.;Ko, M.H.;Ko, C.S.; Kang, H.K.;Biol. Pharm. Bull. 2016, 39, 1273-1283.
CrossRef - Yang, E.J.; Ham, Y.M.; Lee, W.J.; Lee, N.H.; Hyun, C.G.;Daru. 2013, 21, 62.
CrossRef - Seong, Z.K.; Lee, S.Y.;Poudel, A.; Oh, S.R.; Lee, H.K.;Molecules2016, 21, E1296.
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X.; Cosmetics2016, 3, 36.
- Couteau,C.;Coiffard,L.;Cosmetics2016, 3, 27.
- Kim, M.J.; Kim, D.S.; Yoon, H.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G.;Interdiscip.Toxicol.20147, 89-92.
- Ko, R.K.; Kang, M.C.; Kim, S.S.; Oh, T.H.; Kim, G.O.; Hyun, C.G.; Hyun, J.W.; Lee, N.H.;Nat. Prod.Commun.2013, 8, 427-428.

This work is licensed under a Creative Commons Attribution 4.0 International License.









