Antiplatelet aggregation activity of 5-Hydroxyflavone, 2’-Hydroxyflavanone, Paeonol and bergenin isolated from stem bark of Garcinia malaccensis in human whole blood
Khaled.A.A.Alkadi1, Aishah Adam1, Muhammad Taha2, Mizaton Hazizul Hasan2, Syed Adnan Ali Shah1,2*
1Faculty of Pharmacy, Universiti Teknologi MARA (UiTM), Puncak Alam Campus, 42300 Bandar Puncak Alam, Selangor Darul Ehsan, Malaysia. 2Atta-ur-Rahman Institute for Natural Products Discovery, Universiti Teknologi MARA (UiTM), Puncak Alam Campus, 42300 Bandar Puncak Alam, Selangor Darul Ehsan, Malaysia.
DOI : http://dx.doi.org/10.13005/ojc/290304
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
Four known compounds, 5-Hydroxyflavone (1), 2’-Hydroxyflavanone (2), Paeonol (3) and bergenin (4), were isolated from the methanolic extract of the stem bark of Garcinia malaccensis for the first time through combination of vacuum and radial chromatography techniques. The structures of the secondary metabolites (1-4) were elucidated on the basis of their spectroscopic evidence and comparison with the published data. All isolated compounds exhibited inhibition of platelet aggregation in human whole blood which induced by arachidonic acid (AA), adenosine diphosphate (ADP) and collagen. 2’-Hydroxyflavanone (2) showed inhibitory effect on platelet aggregation caused by two inducers with IC50 47.8± 2.1 AA and 147.2 ± 4.1 ADP.
KEYWORDS:Garcinia malaccensis;Platelet aggregation;Impedance Method;vacuum liquid chromatography (VLC);NMR spectroscopy
Download this article as:| Copy the following to cite this article: Alkadi K. A. A, Adam A, Taha M, Hasan M. H, Ali S. A. Antiplatelet aggregation activity of 5-Hydroxyflavone, 2’-Hydroxyflavanone, Paeonol and bergenin isolated from stem bark of Garcinia malaccensis in human whole blood. Orient J Chem 2013;29(3) |
| Copy the following to cite this URL: Alkadi K. A. A, Adam A, Taha M, Hasan M. H, Ali S. A. Antiplatelet aggregation activity of 5-Hydroxyflavone, 2’-Hydroxyflavanone, Paeonol and bergenin isolated from stem bark of Garcinia malaccensis in human whole blood. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=262 |
INTRODUCTION
The plant kingdom has served as one of man’s oldest sources of useful drugs. They are remarkable in their ability to produce a vast number of diverse secondary metabolites ranging in chemical complexity and biological activities. Natural products (NPs) have historically served as templates for the modern drug development. The Garcinia species are known to be rich in phytochemical contents such as flavonoids, phenolic acids and xanthones. G. Malaccensis Hk.f (Guttiferae), locally known as “manggis burung” is a member of the Guttiferae family. Its fruit much resembles that of G. mangostana and G. hombroniana. Its mature fruit is similar in size to G. hombroniana but smaller than G. Mangostana 1. Mangosteen fruits from Garcinia malaccensis (G. Mangostana Hook. f.) Linn also known as the queen of fruit is a most popular xanthone-rich and edible fruit that has been widely used and commercialized for its medicinal properties and is considered to be one of the best of all tropical fruits in Southeast Asia 2-3. Some cytotoxic activities have been reported on the compounds isolated from Garcinia. a-Mangostin from G. mangostana has shown to protect mitochondria from peroxidative damage, apoptotic effects and reducing oxidative damages 4-5.
This paper describes the isolation of four known compounds, 5-Hydroxyflavone (1), 2’-Hydroxyflavanone (2), Paeonol (3) and bergenin (4) from the methanolic extract of the stem bark of Garcinia malaccensis for the very first time using vacuum liquid chromatography (VLC) technique. The identified compounds were exhibited inhibition of platelet aggregation in human whole blood which induced by arachidonic acid (AA), adenosine diphosphate (ADP) and collagen.
MATERIALS AND METHODS
General experimental procedures
Melting points were determined on a Yanaco MP-S3 apparatus. UV spectra were measured on a Shimadzu UV 240 spectrophotometer. JASCO DIP–360 Digital polarimeter was used to measure the optical rotations in chloroform by using 10 cm cell tube. FTIR-8900 Spectrophotometer was used to record IR spectra in CHCl3. The 1H-NMR and 2D NMR spectra were recorded on a Bruker Avance III 500 Ascend spectrometer using BBO probe, while 13C-NMR spectra were recorded on Bruker Avance III 500 Ascend spectrometer operating at 125 MHz using CDCl3 as solvent. Chemical shifts were reported in δ (ppm), relative to SiMe4 as internal standard, and coupling constants (J) were measured in Hz. The EI-MS and HREIMS were measured on Jeol HX 110 mass spectrometer. TLC was performed on Si gel precoated plates (PF254, 20 × 20, 0.25 mm, Merck, Germany). Ceric sulphate in 10% H2SO4 spraying reagent was used for the staining of compounds on TLC. All reagents used were of analytical grades.
Plant material
The stembarks of G. malaccensis were collected from Pasoh, Negeri Sembilan, Malaysia in September 2010. A voucher specimen (MT27) was deposited in the herbarium of the Herbarium of the Forest Research Institute Malaysia (FRIM), Kepong, Malaysia.
Extraction
Dried and powdered stembark of G. malaccensis (500 g) was extracted under reflux with MeOH for 6-8 hrs and filter it. Obtained MeOH extract was fractionate through vacuum liquid chromatography (VLC) technique using different polarity. Evaporation of the respective solvents and purified the compounds through Preparative thin layer chromatography.
Impedance Method
Whole blood (1ml) diluted with buffer saline (1:1) was incubated with samples 5µl,in DMSO)at 37° C for 2 min. After which collagen (2µg/ml), ADP (10µM), AA(o.5 mM) was added to initiate aggregation. The platelet aggregation was measured by whole blood Lumi-Aggregometer (Chrono-Log Corp.Haver-town,PA). The results of aggregation in whole blood was determined after 5 min. as the increase in impedance across a pair of electrodes placed in the blood sample by comparison to that of control group impedance. To eliminate the effect of the solvent on the aggregation, blood with 0.5% DMSO was used as the control. The percentage inhibition of platelet aggregation was calculated as:
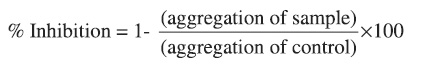
RESULTS AND DISCUSSION
Phytochemical investigation
The dried and powdered stembark of G. malaccensis (500 g) were was extracted under reflux with MeOH for 6-8 hrs and filter it. Obtained MeOH extract was fractionate through vacuum liquid chromatography (VLC) technique using different polarity. Evaporation of the respective solvents and purified the four compounds (1–4) through Preparative thin layer chromatography (Figure 1). All spectroscopic data described below were showed excellent agreement with the previously published results 6-9.
5-Hydroxyflavone (1) was obtained as a yellow crystal (8.2 mg); mp. 175-176° C. Rf 0.23 in MeOH; UV (MeOH) lmax (log e) 240 (2.3) nm; IR (CHCl3) nmax 3432, 2152, 1690 and 1670 cm-1; EIMS for C15H10O3 m/z (rel. int.): 238[M]; 1H NMR (CDCl3, 500 MHz): δ 6.71, 6.79, 6.97, 7.54, 7.88, 7.89, 12.57. 13C NMR (CDCl3, 125 MHz): δ 135.6(C-1), 126.6(C-2’, 6’), 129.3(C-3’, 4’, 5’), 77.3(C-2), 183.7(C-4), 161.0(C-4a), 164.7 (C-5), 156.6(C-6).
2’-Hydroxyflavanone (2) was obtained as a white yellowish powder (7.3 mg); mp. 178-180° C. Rf 0.30 in MeOH; UV (MeOH) lmax (log e) 235 (2.41) nm; IR (CHCl3) nmax 3412, 2152, 1680 and 1660 cm-1; EIMS for C15H12O3 m/z (rel. int.): 240[M]; 1H NMR (Acetone-d6, 500 MHz): δ 2.93, 3.09, 5.85, 6.98, 7.11, 7.23, 7.56, 7.88, 8.80. 13C NMR (Acetone-d6, 125 MHz): δ 29.2, 43.2, 75.1, 115.6, 118.2, 120.0, 121.2, 121.5, 126.0, 127.0, 129.5, 136.0, 154.1, 162.2, 191.7.
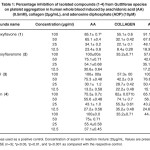 |
Table 1: Percentage inhibition of isolated compounds (1-4) from Guttiferae species on platelet aggregation in human whole blood induced by arachidonic acid (AA) (0.5mM), collagen (2µg/mL), and adenosine diphosphate (ADP) (10µM). Click here to View table |
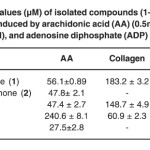 |
Table 2: IC50 values (µM) of isolated compounds (1-4) on platelet aggregation induced by arachidonic acid (AA) (0.5mM), Collagen (2µg/ml), and adenosine diphosphate (ADP) (10µM). Click here to View table |
Paeonol (3) was obtained as a white powder (5.5 mg); mp. 175-179° C. Rf 0.42 in MeOH; UV (MeOH) lmax (log e) 233 (2.32) nm; IR (CHCl3) nmax 3443, 2121, 1680 and 1663 cm-1; EIMS for C9H10O3 m/z (rel. int.): 166[M]; 1H NMR (CDCl3, 500 MHz): δ 2.54 (3H, s), 3.82 (3H, s), 6.40 (1H, d), 6.42 (1H, d), 7.60 (1H, d), 12.75 (1H, s). 13C NMR (CDCl3, 125 MHz): δ 114.0 (C-1), 165.4 (C-2), 100.9 (C-3), 166.2 (C-4), 107.7 (C-5), 132.5 (C-6), 202.7 (COCH3), 26.3 (COCH3), 55.7 (OCH3).
Bergenin (4) was obtained as a brownish powder (6.2 mg); mp. 183-185° C. Rf 0.40 in MeOH; UV (MeOH) lmax (log e) 243 (2.67) nm; IR (CHCl3) nmax 3452, 2120, 1684 and 1665 cm-1; EIMS for C14H16O9 m/z (rel.int.): 328[M]; 1H NMR (DMSO-d6, 500 MHz): δ 3.56 (1H, dd), 3.44 (1H, dd), 3.64 (1H, dd), 3.98 (1H, dd), 6.95 (1H, s), 4.98 (1H, d), 3.96 (1H, d), 3.76 (3H,s). 13C NMR (DMSO-d6, 125 MHz): δ 81.7(C-2), 70.7(C-3), 73.7(C-4), 79.8(C-4a), 163.4(C-6), 118.1(C-6a), 109.5(C-7), 151.0(C-8), 140.6(C-9), 148.1(C-10), 116.0(C-10a) , 72.1(C-10b), 61.1(C-11), 59.9(C-12).
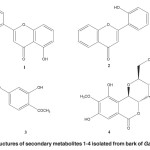 |
Figure 1: Chemical structures of secondary metabolites 1-4 isolated from bark of Garcinia malaccensis. Click here to View figure |
Antiplatelet aggregation activity
The effect of the isolated compounds (1–4) have been investigate against platelet aggregation test. Each sample was measured in triplicate one-way analysis of variance (ANOVA) was used for multiple comparison, the IC50 values, that is, the concentration of the compounds required to inhibit aggregation by 50% ,were obtained from at least three determinations7. Methanol extract of Garcinia malaccensis showed strong antiplatelet aggregation activity at 100µg/ml in human whole blood in vitro. Both 5-Hydroxyflavone (1), Paeonol (3) and Bergenin (4) showed marked inhibitory effect on platelet aggregation caused by the three inducers. The IC50 value for 5-Hydroxyflavone (1) was 56.1±0.89AA, 183.2 ± 3.2 Collagen and 69.7±0.76c ADP, the IC50 value for Paeonol (3) was 47.4 ± 2.7 AA, 148.7 ± 4.9 Collagen and 61.9 ± 1.1 ADP and the IC50 value for Bergenin (4) was 240.6 ± 8.1 AA, 60.9 ± 2.3 Collagen and 120.3 ± 5.2 ADP, 2’-Hydroxyflavanone (2) showed inhibitory effect on platelet aggregation caused by two inducers with IC50 47.8± 2.1 AA and 147.2 ± 4.1 ADP (table 1 & table 2).
ACKNOWLEDGMENTS
The author wishes to acknowledge Faculty of Pharmacy, Universiti Teknologi MARA Puncak Alam campus for financial support.
REFERENCES
- Y. Nakagawa, M. Iinuma, T. Naoe, Y. Nozawa and Y. Akao, Bioorg. Med. Chem. 15(16): 5620-5628 (2007).
- B. Márquez-Valadez, R. Lugo-Huitrón, V. Valdivia-Cerda, L. R. Miranda-Ramírez, V, Pérez-De La Cruz, O, González-Cuahutencos, Nutr. Neurosci. 12(1): 35-42 (2009).
- J. Pedraza-Chaverri, N. Cárdenas-Rodríguez, M. Orozco-Ibarra and J. M. Pérez-Rojas, Food Chem. Toxicol. 46(10): 3227-3239 (2008).
- M. Taher, D. Susanti, M. F. Rezali, F. S. A. Zohri, S. J. A. Ichwan, S. I. A, F. Ahmad, Asian Pacific Journal of Tropical Medicine. 136-141 (2012).
- E. Martínez-Abundis, N. García, F. Correa, S. Hernández-Reséndiz, J. Pedraza-Chaverri and C. Zazueta, Mitochondrion. 10(2): 151-157 (2010).
- A. Wibowo, N. Ahmat, A.S. Hamzah, A.S. Sufian, N.H. Ismail, R. Ahmad, F.M. Jaafar and H. Takayama, Fitoterapia. 82: 676–681 (2011).
- P. N. Kim Tuyen, L. H. Duy and K. P. P. Nguyen, Tap Chi Hoa Hoc. 48(5): 546-550 (2010).
- J. F. Stevens, M. Ivancic, M. L. Deinzer and Eckhard Wollenweber, J. Nat. Prod. 62 (2): 392–394 (1999).
- F. A. Toma´s-Barbera´n, E. Wollenweber, Plant Syst. Evol. 173: 109-118 (1990).
- N, Kabakuş, B, Yilmaz, U, Calişkan, Haematologia (Budap). 30(2): 107-115 (2000).

This work is licensed under a Creative Commons Attribution 4.0 International License.









