Acanthopanax koreanum roots inhibit the expression of pro-inflammatory cytokines, inducible nitric oxide synthase, and cyclooxygenase-2 in RAW 264.7 macrophages
Eun-Jin Yang1, Kwang Hee Hyun2, Hyun Kim2, Min-Jin Kim1, Nam Ho Lee1, and Chnag-Gu Hyun1*
1Cosmetic Sciences Center, Department of Chemistry and Cosmetics, Jeju National University, Jeju 690-756, Korea 2Helios Co., Ltd., Sanchundan Dong-gil 16, Jeju 690-121, Korea Corresponding autho E-mail: cghyun@jejunu.ac.kr
DOI : http://dx.doi.org/10.13005/ojc/320103
Article Received on :
Article Accepted on :
Article Published : 03 Mar 2016
Acanthopanax koreanum is a popular plant found on Jeju Island, Korea and is commonly used to prevent the side effects of consumption of alcoholic beverages. However, this plant has not been properly utilized as a medicinal material. In this study, we investigated the anti-inflammatory effects of the 70% ethanol extract of A. koreanum roots (AKR-E). The results indicated that the AKR-E (200 μg/mL) inhibited the lipopolysaccharide (LPS)-induced production of nitric oxide (NO) and prostaglandin E2 (PGE2) in RAW 264.7 macrophages by 41.2% and 78.9%, respectively. These effects were accompanied by concentration-dependent decreases in the expression levels of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) proteins. Additionally, the AKR-E inhibited the expression of pro-inflammatory cytokines, including interleukin (IL)-6 (22.7%) and IL-1β (74%). These data showed that the AKR-E had protective effects against the induction of LPS-induced inflammation in RAW 264.7 macrophages.
KEYWORDS:Acanthopanax koreanum roots; cyclooxygenase-2, inducible nitric oxide synthase; interleukin-6; interleukin-1β
Download this article as:| Copy the following to cite this article: Yang E.J, Hyun K. H, Kim H, Kim M. J, Lee n. h, Hyun C. G. Acanthopanax koreanum roots inhibit the expression of pro-inflammatory cytokines, inducible nitric oxide synthase, and cyclooxygenase-2 in RAW 264.7 macrophages. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Yang E.J, Hyun k. h, Kim H, Kim M. J, Lee n. h, Hyun C. G. Acanthopanax koreanum roots inhibit the expression of pro-inflammatory cytokines, inducible nitric oxide synthase, and cyclooxygenase-2 in RAW 264.7 macrophages. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14554 |
Introduction
Acanthopanax species are widely distributed throughout Korea, China, and Japan. In particular, Acanthopanax koreanum Nakai (Araliaceae), an indigenous plant of Jeju Island in South Korea, has been used for the treatment of rheumatism, diabetes, and hepatitis1-3. Recent studies have suggested that A. koreanum has protective effects against severe hepatitis induced by lipopolysaccharide (LPS) and d-galactosamine (d-GalN) in mouse and rat models4,5.
Inflammation, which is characterized by heat, swelling, fever, and pain, is an important defensive mechanism that functions to alleviate injury and protect against infection6,7. Macrophages, a main component of the inflammatory pathway, are involved in the early response to LPS, a component of the outer membrane of gram-negative bacteria and a ligand of Toll-like receptor 4 (TLR4) that activates a variety of immunological responses. Macrophages infected early response to LPS, and also play a pivotal role in host defense and homeostasis. However, continuous stimulation of macrophages by LPS results in cell death and excessive secretion of inflammatory mediators, such as nitric oxide (NO), prostaglandin E2 (PGE2), interleukin (IL)-6, and IL-1β8-12. Moreover, in previous studies, we demonstrated the anti-inflammatory effects of A. koreanum fruit waste on LPS-induced RAW 264.7 macrophages and showed that this mechanism was dependent on inhibitor of kappa B alpha (IκB-α)1. However, the anti-inflammatory effects of A. koreanum roots have not yet been proven.
In this study, we examined whether treatment with A. koreanum roots could inhibit the LPS-induced expression of inflammatory mediators and pro-inflammatory cytokines, such as NO, PGE2, IL-6, and IL-1β in RAW 264.7 macrophages.
Materials and Methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). LPS (Escherichia coli 0111:B4) was purchased from Sigma Aldrich (St. Louis, MO, USA). All other chemicals used were of analytical grade. IL-6 (BD Bioscience, Mountain View, CA, USA), PGE2, and IL-1β (R&D Systems, St. Louis, MO, USA) in the supernatants of cultured RAW 264.7 macrophages were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions. Antibodies against iNOS were purchased from Calbiochem (San Diego, CA, USA), and antibodies against COX-2 were purchased from BD Biosciences (San Diego, CA, USA).
Cell culture
Murine RAW 264.7 macrophages were purchased from the Korean Cell Line Bank (Seoul, Korea). Cells were cultured in DMEM containing 2 mM glutamine, 10 mM HEPES, penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% FBS. Cells were cultured at 37°C in a humidified incubator containing 5% CO2.
Dimethylthiazol-2yl)-,5-diphenyltetrazolium bromide (MTT) assays for cell viability
Cell viability was determined using MTT assays13,14. RAW 264.7 cells were cultured in 24-well plates for 18 h, followed by treatment with various concentrations (50, 100, 150, or 200 μg/mL) of the EtOH extract of A. koreanum roots (AKR-E) for 24 h. Briefly, MTT was added to cells and the formazan crystals were dissolved in dimethyl sulfoxide (DMSO). The absorbance was measured at 540 nm. The percentage of cells showing cytotoxicity was determined relative to that in the control group.
NO assays
NO accumulation was used as an indicator of NO production in the cell culture medium using Griess reagent15,16. The culture supernatant (100 μL) was mixed with the same volume of Griess reagent (1% sulfanilamide and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride in 5% phosphoric acid) for 10 min, and absorbance was measured at 540 nm.
Western blot analysis
After incubation for 24 h, the cells washed twice with cold phosphate-buffered saline (PBS), lysed in lysis buffer (RIPA buffer, 1% Nonidet P-40, 1% protease inhibitor cocktail), and kept on ice for 30 min. The cell lysates were then centrifuged at 15,000 rpm for 15 min at 4°C. The protein concentrations were measured using the Branford method. The cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 8–12% gels and electrotransferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% skim milk overnight at 4°C and then incubated for 2 h at room temperature with a 1:2000 dilution of the primary antibody. After washing, the membranes were incubated 30 min at room temperature with a 1:5000 dilution of horseradish peroxidase-conjugated secondary antibody. The proteins were then detected using a WEST-ZOL Western Blot Detection System (iNtRON, Gyeonggi, Korea).
Data analysis
All data were expressed as the means ± standard deviations of at least replicates. Student’s t-tests and one-way analysis of variance (ANOVA) were used for statistical analyses, and differences with P values of less than 0.05 were considered significant.
Results and Discussion
The AKR-E did not trigger cytotoxicity in RAW 264.7 macrophages
RAW 264.7 macrophages were treated with various concentrations of the AKR-E for 24 h, and cell viability was assessed using MTT assays. Notably, when used at concentrations ranging from 50 to 200 μg/mL, the AKR-E did not induce cytotoxicity in the cells compared with that in the untreated control cells (Figure 1). However, at higher concentrations, the AKR-E was cytotoxic (data not shown). Thus, 200 μg/mL AKR-E was selected for further experiments.
LPS-induced production of NO and PGE2 was markedly inhibited decreased iNOS and COX-2 protein expression following treatment with the AKR-E
Macrophages play a critical role in the active and passive immune responses and control a variety of inflammatory mediators, including NO, PGE2, and cytokines. Among these mediators, NO plays an important role in eliminating tumors and bacterial infections. However, excessive NO formation by pathological mechanisms can result in inflammation. Moreover, excessive production of NO by
overexpression of iNOS is associated with several inflammatory disorders, including septic shock and rheumatoid arthritis17-19.
The inhibitory effects of the AKR-E against LPS-induced NO production and iNOS protein expression were assessed by Griess reagent assays and western blotting in RAW 264.7 macrophages. After treatment for 24 h, 1 μg/mL LPS induced iNOS expression and subsequent production of NO. Notably, treatment with AKR-E (25, 50, 100, or 200 μg/mL) reduced the level of NO production and iNOS protein expression in a concentration-dependent manner (Figures 1 and 2).
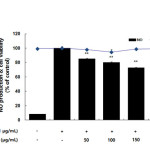 |
Figure 1: Effects of the AKR-E on nitric oxide production in LPS-stimulated RAW264.7 macrophages. The cells were stimulated with 1 µg/mL LPS only or with LPS plus various concentrations (50, 100, 150, or 200 µg/mL) of the AKR-E for 24 h. Nitric oxide production was determined by the Griess reagent method. Cell viability was determined from the 24 h culture of cells stimulated with LPS (1 µg/mL) in the presence of the AKR-E. The data represent the means ± SDs of triplicate experiments. *P < 0.05, **P < 0.01 versus LPS alone. Click here to View figure |
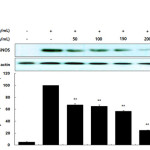 |
Figure 2. Effects of the AKR-E on the activation of iNOS in LPS-stimulated RAW 264.7 macrophages. RAW 264.7 macrophages (5.0 × 105 cells/mL) were stimulated with LPS (1 μg/mL) in the AKR-E (50, 100, 150, or 200 μg/mL) for 24 h. Whole-cell lysates (25 μg) were prepared, and the proteins were separated by SDS-PAGE. The expression of iNOS and β-actin was determined by western blotting. Click here to View figure |
Many anti-inflammatory drugs inhibit prostaglandin synthesis through inhibition of COX-2. PGE2 is generated from arachidonic acid by COX enzymes, including COX-1 and COX-2. COX-1 mediates prostaglandin synthesis to regulate gastrointestinal and renal function and platelet formation. In contrast, COX-2 expression is induced by growth factors, mitogens, and cytokines and has been shown to induce inflammation-related diseases by facilitating the production of large amounts of prostaglandins. Thus, prostaglandins produced by COX-2 are thought to mediate the inflammatory response20-22. In this study, we showed that LPS significantly stimulated PGE2 production and that the AKR-E effectively inhibited the production of PGE2 in RAW 264.7 macrophages (Figure 3). Moreover, analysis of COX-2 protein expression confirmed that COX-2 was markedly downregulated (Figure 4).
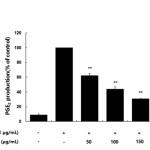 |
Figure 3. Effects of the AKR-E on PGE2 production in LPS-stimulated RAW 264.7 macrophages. The cells were stimulated with 1 μg/mL LPS only or with LPS plus various concentrations (50, 100, 150, or 200 μg/mL) of the AKR-E for 24 h. PGE2 production was assayed by ELISA. The data represent the means ± SDs of triplicate experiments. *P < 0.05, **P < 0.01 versus LPS alone. Click here to View figure |
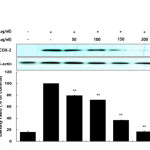 |
Figure 4: Effects of AKR-E on the activation of COX-2 in LPS-stimulated RAW 264.7 macrophages. RAW 264.7 macrophages (5.0 × 105 cells/mL) were stimulated with LPS (1 μg/mL) in the hexane fraction of A. koreanum (50, 100, 150, or 200 μg/mL) for 24 h. Whole-cell lysates (25 μg) were prepared, and the proteins were separated by SDS-PAGE. The expression of COX-2 and β-actin was determined by western blotting. Click here to View figure |
LPS-induced production of IL-6 and IL-1β was inhibited by the AKR-E
Inflammatory mediators, such as IL-6 and IL-1β, have been regulate inflammatory responses in both in vivo and in vitro, and these cytokines are the major pro-inflammatory cytokines produced in macrophages. IL-6 is involved in the primary immune response, and IL-1β is a key cytokine involved in the initiation and enhancement of the inflammatory response to bacterial infection. These cytokines are known to interact with each other and are generated in response to LPS stimulation23-25. In this study, we found that treatment with LPS induced the expression of both IL-6 and IL-1β and that subsequent treatment with the AKR-E significantly blocked these increases (Figures 5 and 6).
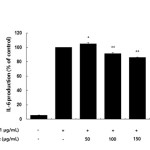 |
Figure 5: Effects of the AKR-E on IL-6 production in LPS-stimulated RAW 264.7 macrophages. The cells were stimulated with 1 μg/mL LPS only or with LPS plus various concentrations (50, 100, 150, or 200 μg/mL) of the AKR-E for 24 h. IL-6 production was assayed by ELISA. The data represent the means ± SDs of triplicate experiments. *P < 0.05, **P < 0.01 versus LPS alone. Click here to View figure |
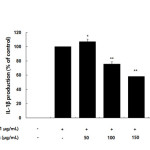 |
Figure 6. Effects of the AKR-E on IL-1β production in LPS-stimulated RAW 264.7 macrophages. The cells were stimulated with 1 μg/mL LPS only or with LPS plus various concentrations (50, 100, 150, or 200 μg/mL) of the AKR-E for 24 h. IL-1β production was assayed by ELISA. The data represent the means ± SDs of triplicate experiments. *P < 0.05, **P < 0.01 versus LPS alone. Click here to View figure |
Conclusion
In summary, the AKR-E inhibited the production of NO and PGE2 and the expression of iNOS and COX-2 protein in RAW 264.7 macrophages. In addition, the AKR-E inhibited the production of pro-inflammatory cytokines, such as IL-6 and IL-1β. Therefore, these results supported that the roots of A. koreanum may have applications as an anti-inflammatory agent, providing insights into the mechanisms of the anti-inflammatory effects of the AKR-E.
Conflict of interest
There is no conflict of interest.
Financial support
This study was supported by the Innovative Fusion R&D Program for regional industry promotion through the Korea Institute for Advancement of Technology funded by the Ministry of Trade, Industry, and Energy (R0004214).
References
- Yang, E.J.; Moon, J.Y.; Lee J.S.; Koh J.; Lee N.H.; Hyun C.G.; Journal of Biomedicine and Biotechnology, 2010. 2010, 715739.
- Kang, H.S.; Kim, Y.H.; Lee, C.S.; Lee, J.J.; Choi, I.; Pyun, K.H.; Cellular Immunology . 1996, 170, 212-221.
CrossRef - Nan, J.X,; Jin, X.J.; Lian, L.H.; Cai, X.F.; Jiang, Y.Z.; Jin, H.R.; Lee, J.J.; Biological and Pharmaceutical Bulletin, 2008, 31, 738-742.
CrossRef - Nan, J.X,; Park, E.J.; Nam, J.B.; Zhao, Y.Z.; Cai, X.F.; Kim, Y.H.; Sohn, D.H.; Lee, J.J.; Journal of Ethnopharmacology, 2004, 92, 71-77.
CrossRef - Jung, M.G.; Do, G.M.; Shin, J.H.; Ham.,Y.M.;, Park, S.Y.; Kwon, O.; Nutrition Research and Practice, 2013, 7, 460-465.
CrossRef - Hou, D.X.; Masuzaki, S.; Hashimoto, F.; Uto, T.; Tanigawa, S.; Fujii, M.; Sakata, Y.;. Archives of Biochemistry and Biophysics, 2007, 460, 67-74.
CrossRef - Kim, J.B.; Han, A.R.; Park, E.Y.; Kim, J.Y.; Cho, W.; Lee, J.; Seo, E.K.; Lee, K.T.; Biological and Pharmaceutical Bulletin, 2007, 30, 2345-2351.
CrossRef - Guha, M.; Mackman, N.; Cellular Signalling, 2001, 13, 85-94.
CrossRef - Kiyoshi, Takeda.; Shizuo, Akira.; Annual Review of Immunology, 2005, 23, 307-335.
- McDaniel, M.L.; Kwon, G.; Hill, J.R.; Marshall, C.A.; Corbett, J.A.; Proceedings of the Society for Experimental Biology and Medicine, 1996, 211, 24-32.
CrossRef - Meng, F.; Lowell, C.A.; The Journal of Experimental Medicine, 1997, 185, 1661-1670.
CrossRef - Willeaume, V.; Kruys, V.; Mijatovic, T.; Huez, G.; Journal of Inflammation, 1995, 46, 1-12.
- Gerlier, D.; Thomasser, N.; Journal of Immunological Methods, 1986, 94, 57-63.
CrossRef - Liu, Y.; Progress in Neuro-Psychopharmacology and Biological Psychiatry, 1996, 23, 377-395.
CrossRef - Snell, J.C.; Colton, C.A.; Chernvshev, O.N.; Gilgert, D.L.; Free Radical Biology and Medicine, 1996, 20, 361-363.
CrossRef - Woo, E.R.; Lee, J.Y.; Cho, I.J.; Kim, S.G.; Kang, K.W.; Pharmacological Research, 2005, 51, 539-546.
CrossRef - Choi, C.Y.; Park, K.R.; Lee, J.H.; Jeon, Y.J.; Liu, K.H.; Oh, S.; Kim, D.E.; Yea, S.S.; European Journal of Pharmacology, 2007, 576, 151-159.
CrossRef - Aldridge, C.; Razzak, A.; Babcock, T.A.; Helton, W.S.; Espat, N.J.; The Journal of Surgical Research, 2008, 149, 296-302.
CrossRef - Shellito, J.E.; Kolls, J.K.; Summer, W.R.; American Journal of Respiratory Cell and Molecular Biology, 1995, 13, 45-53.
CrossRef - Milano, S.; Arcoleo, F.; Dieli, M.; D’Agostino, R.; D’Agostino, P.; De, Nucci, G.; Cillari, E.; Prostaglandins, 1995, 49, 105-115
CrossRef - Lin, W.W.; Chen, B.C.; Hsu, Y.W.; Lee, C.M.; Shyue, S.K.; Prostaglandins and Other Lipid Mediators, 1999, 58, 87-101.
CrossRef - Eliopoulos, A.G.; Dumitru, C.D.; Wang, C.C.; Cho, J.; Tsichlis, P.N.; The EMBO Journal, 2002, 21, 4831-4840.
CrossRef - Beuscher, H.U.; Günther, C.; Röllinghoff, M.; Journal of Immunology, 1990, 144, 2179-2183.
- Jang, C.H.; Choi, J.H.; Byun, M.S.; Jue, D.M.; Rheumatology (Oxford), 2006, 45, 703-710.
CrossRef - Wan, Y.; Freeswick, P.D.; Khemlani, L.S.; Kispert, P.H.; Wang, S.C.; Su, G.L.; Billiar, T.R.; Infection and Immunity, 1995, 63, 2435-2442.

This work is licensed under a Creative Commons Attribution 4.0 International License.









